Subnanometer wide indium selenide nanoribbons
March 14, 2023 - Indium selenides have been shown to retain several desirable properties, such as ferroelectricity and high electron mobility, when confined to two dimensions (2D). A team of researchers from the Universities of Nottingham (United Kingdom) and Ulm (Germany), as well as the National Academy of Sciences of Ukraine and the Diamond Light Source (United Kingdom), has identified new ways to control the phase of sub-nm InxSey nanoribbons that could be important for versatile future nanoelectronic devices.
Indium selenides (InxSey) belong to a family of group III-VI semiconductors that are attracting increasing research interest due to their potential application as ultrathin and flexible components of photovoltaic and optoelectronic devices.[1, 2] These materials are known to exist in a wide variety of stoichiometric ratios and, among them, in different phases with different chemical and physical properties. Researchers have been conducting studies on the transformation of stoichiometries, phases, and stacking of InxSey compounds, often with conflicting results.[3] If their full potential is to be realized, an understanding of the complex polymorphism of InxSey must be established. Beyond the known members of the InxSey family, computational studies based on swarm intelligence predict the existence of experimentally undiscovered stable polymorphs of InSe, indicating the importance of further research to control the structure of InxSey compounds.[4]
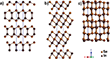
Indium sesquiselenide (InxSey) is known to exist in five different phases, with α-, β-, and γ-phases being the most common.[5] In α-In2Se3, half of the In atoms are octahedrally coordinated and half are tetrahedrally coordinated, while in β-In2Se3 all of the In atoms are octahedrally coordinated. Both α- and β-In2Se3 phases are layered structures in which van der Waals bonds separate the layers in the c-axis direction, and bulk phases of both are known to undergo irreversible phase transitions to γ-In2Se3 at high temperatures. The exact temperature required for this transformation is controversial, but it has been shown to occur between 350 and 650 °C.[6] Unlike its α- and β-counterparts, γ-In2Se3 is not a layered structure but forms a defective wurtzite crystal structure. Indium monoselenide (InSe) can exist in three different phases, β, ε, or γ, which are determined by the stacking of van der Waals layers in the structure.[7] The structures of β-In2Se3, γ-In2Se3, and γ-InSe are shown in Figure 1.
The observation of quantum Hall effects at room temperature and liquid helium has also been reported in atomically thin films of γ-InSe, suggesting that they may be used for high-quality 2D semiconductor components in the future.[8] Confining the phases of In2Se3 to 2D has shown that certain unique properties of the bulk material can be retained, including room-temperature ferroelectricity and high electron mobility.[6] Ferroelectricity in few and single layers of α-In2Se3 was predicted theoretically by Ding et al. with subsequent experimental studies confirming the same property in few layers of α-In2Se3 and β-In2Se3.[9]
Despite advances in the field, nanoscale fabrication of InxSey is generally not scalable, often relying on liquid phase or mechanical exfoliation to create 2D layers. In2Se3 nanowires have been successfully synthesized, demonstrating that the complicated phase change behavior of InxSey compounds is preserved when fabricated as templates in this manner, along with high photosensitivity and rapid photoreaction when used as visible light photodetectors.[10, 11] However, the nanowires have a much larger diameter than a single van der Waals layer (between 40 and 200 nm), which means that they lack some of the exciting properties of ultrathin, single-layer InxSey. In 2021, low-dimensional In2Se3 showed that the smallest In2Se3 nanowires synthesized were still 40-80 nm in diameter.[10, 12] In addition, it was shown that low-dimensional InxSey field-effect transistors (FETs) are damaged by oxidation under ambient conditions if they are not protected by a passivating hexagonal boron nitride layer, which is an important consideration if widespread use of InxSey -based nanoelectronic components.[13]
Researchers have now used the highly anisotropic geometry of SWCNTs to grow InxSey and study the material using transmission electron microscopy (TEM). The nanoribbons provides a unique environment for the templated growth of ultrathin nanomaterials[14] and their study at the atomic scale.[15] Recently synthesized materials include ReS2,[16] SnSe,[17] and HgTe,[18] each of which forms ultrathin nanoribbons inside SWCNTs. Nanowires and nanoribbons synthesized by encapsulation in SWCNTs have great potential as components in nanoelectronic devices due to their high aspect ratio and small diameter.[19] SWCNTs also provide enhanced thermal and chemical stability to the encapsulated materials, as they have been shown to increase the melting point and/or prevent decomposition under ambient conditions compared to their unencapsulated counterparts.[20] In addition to providing a stable nanoscale container, the extreme confinement enforced by encapsulating a molecule or molecular material in SWCNTs has been shown to produce unique species with geometries and bonds not seen in their bulk counterparts.[21,22]
Ultrathin nanoribbons inside SWCNTs from melt growth
Melt growth has already been used to successfully encapsulate a variety of compounds in CNTs, including metal chlorides, metal oxides, and elemental species, enabling the synthesis of numerous ultrathin nanowires and nanoribbons.[23,24] "We have investigated two methods for the synthesis of InxSey nanoribbons, both using 1.5 nm diameter SWCNTs as templates" say the authors. "The first method is based on melt growth: Melting of γ-InSe in the presence of open SWCNTs."
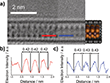
Due to their extremely narrow width and lack of 3D periodicity, conventional spectroscopy and diffractometry methods proved insufficient to characterize the encapsulated nanoribbons. Therefore, the scientists applied local probe microscopy methods, including conventional HRTEM and AC-HRTEM, to determine the structure and composition of the InxSey nanoribbons. The atomically thin sidewalls of SWCNTs can be readily penetrated by the electron beam of a transmission electron microscope (TEM), enabling the acquisition of atomically resolved direct space images. Conventional HRTEM analyses of the filled SWCNTs revealed that the encapsulated nanoribbons exhibit a high degree of translational mobility within the SWCNT inner cavity due to the influence of the electron beam (see Figure 2), making it difficult to accurately determine the atomic positions and thus the chemical structure of the encapsulated nanoribbons. Such rapid translational motion has not been reported for metal sulfide nanoribbons in carbon nanotubes.[25] It is likely related to repulsive interactions between the terminal Se atoms at the edges of the InSe nanowire and the carbon atoms of the SWCNT inner cavity, as predicted previously for Se chains on graphitic carbon.[26] However, the scientists found that the encapsulated nanoribbons could be atomically resolved by either increasing the acquisition rate of TEM imaging or finding areas where the nanoribbons were fixed by small defects in the SWCNT sidewalls. AC-HRTEM examination of these areas provided atomic-resolution images of the encapsulated nanoribbons, which provided information about the structure of the encapsulated species. It is important to note that the phase of InSe (e.g., β, ε, or γ) only determines differences in stacking between multiple van der Waals layers of the crystal. Consequently, classification of the exact phase of encapsulated InSe is unnecessary when the encapsulated nanoribbon consists of a single monolayer, as is the case here.
The AC TEM image shown in Figure 2 (blue box) shows a nanoribbon that looks similar in projection to a γ-InSe monolayer in the (110) plane, as highlighted in the blue box. The similarities in appearance were further investigated by a comparative contrast analysis of experimental and simulated TEM images of the (110) plane of InSe@SWCNT, where very similar distances (0.34 and 0.35 nm, respectively) between the atomic columns were found (Figure 3b-d). Moreover, the width of the nanoribbon in the (110) plane was experimentally measured to be 0.67 nm, which agrees well with the simulated width of 0.68 nm. The nanoribbon shown in Figure 2, shown in the yellow box, is rotated 90° about the SWCNT axis and has a projection similar to the (001) plane of a γ-InSe monolayer (see yellow box). TEM simulations were used to confirm this similarity, with a matching diameter (0.62 nm experimental and 0.62 nm simulated) and alternating atomic spacings in both the simulated and experimental images, as shown in Figure 3f-h. The close agreement between the simulated and experimental TEM images for the two different orientations of the same nanoribbon is compelling evidence for the successful encapsulation of single-layer InSe in SWCNT.
Ultrathin nanoribbons inside SWCNTs from Stepwise Synthesis
The second method that the scientists employed is a stepwise nanoribbon synthesis in which selenium and indium precursors are sequentially introduced into the nanotubes, similar to the previously reported synthesis of SWCNT-encapsulated metal sulfides.[25] Recent work by Huang et al. has shown that InSe nanoflocks can be synthesized from Se and InI vapor using an Ar and H2 carrier gas.[27] It has been shown that the H2 contained in the carrier gas mixture promotes the production of InSe via In2Se3 and induces the growth of multiple InSe layers. For the synthesis of InxSey @SWCNT, metallic Se and InCl were chosen as Se and In precursors, respectively. InCl was chosen instead of InI because it has higher solubility in conventional solvents such as water and THF, so that externally adsorbed precursors can be easily removed in subsequent washing steps. InCl and Se were found to sublime at 350 and 550 °C, respectively, when sealed under a vacuum of 10−5 mbar. In addition, no hydrogen carrier gas was used because there was no need to create multiple layers of InxSey while inducing the formation of In2Se3 over InSe. Selenium was first introduced into SWCNTs, followed by selective removal of excess selenium from the outer surface of the nanotube by washing with CS2. Subsequently, InCl was sublimed into the nanotubes, inducing the reaction with Se encapsulated in SWCNTs according to the equation.
3InCl(g)+3/8Se8(s)→In2Se3(s)+InCl3(g)
Similar to the encapsulated nanoribbons grown from the melt, AC-HRTEM analysis at 80 kV was performed to determine the exact phase of the stepwise In2Se3 and to show the high translational mobility of the encapsulated nanoribbons. Figure 4a-d shows a nanoribbon whose appearance, atomic spacing, and nanoribbon diameter are very similar to that of a simulated monolayer of β-In2Se3 viewed along the (110) plane and progressing in the (100) plane. Other orientations of encapsulated β-In2Se3 were also observed, including nanoribbons with an appearance like the monolayer β-In2Se3 in the (100) plane, rotated 40° about the axis of the SWCNT (Figure 4e-h). Note that the SWCNT likely acts as a protective shell for the encapsulated In2Se3 throughout the experiment. As noted by Bendall et al, SWCNT-encapsulated metal halides are much more thermally stable than their unencapsulated counterparts.[28] X-ray powder diffraction (PXRD) analysis of the purified In2Se3 @SWCNTs, performed after TGA, revealed the presence of NiO (residual catalyst from nanotube growth) and In2O3 , a combustion product of In2Se3. It can be assumed that 1.1% of the residual mass of the residual weight burned during TGA is NiO. Assuming complete combustion, this corresponds to a 5.0 wt% loading of In2Se3 within the SWCNTs.[29] This percentage loading is similar to other CNT-encapsulated species.[30]
At 400 °C, there was a visible change in the structure of the encapsulated nanoribbons; the AC-TEM images before and after heating are shown in Figure 5. The scientists also found that the mobility of the encapsulated nanoribbons decreased dramatically after heating the nanoribbon. To better understand the nature of this transformation, a small section of the nanoribbon was selected for further analysis, indicated by red boxes in Figure 5. Coincidentally, two fullerene-like particles of amorphous carbon, ubiquitous inside SWCNTs as a byproduct of their synthesis and marked with blue asterisks in Figure 5, were found as "caps" of a small nanoribbon of βIn2Se3, which could be used as reference points before and after heating.
Figure 6a-c shows the nanoribbon at lower temperature in the experimental AC TEM images compared to the simulated TEM images, which show a very similar contrast pattern and nanoribbon diameter in the projection. To rule out the possibility that the heating-induced structural changes are merely a rotation of the β-In2Se3 nanoribbon in the axis of the SWCNT, a rotation series simulating the rotation of β-In2Se3 along the (100)-plane was created as viewed. No projections of the rotated (100)-plane β-In2Se3 are consistent with the appearance of the nanoribbons at high temperatures (Figure 6b), indicating a thermally induced phase change. It is known that β-In2Se3 undergoes an irreversible phase transition to γ-In2Se3 at high temperatures. The exact temperature required for this transformation is controversial, but it has been reported to occur between 350 and 650 °C.[6] The researchers hypothesized that the same phase transition is observed here that converts nano-dense β-In2Se3 to γ-In2Se3. With this in mind, the crystal structure of γ-In2Se3 was used as a starting point to fabricate a nanowire with the correct diameter and a similar appearance in projection to the nanowire shown in Figure 5b at higher temperatures. They found that the simulated TEM images of a nanowire fabricated from γ-In2Se3, viewed along the (120) plane, had a complementary appearance to the experimental AC TEM images, as shown in Figure 7a-c. Again, a "repeat unit" for a nanowire of γ-In2Se3 was constructed from the truncated crystal structure of γ-In2Se3. It is important to note that because the bulk of γ-In2Se3 has a lattice in which the atoms are covalently bonded in all three dimensions, the resulting encapsulated structures of γ-In2Se3 should be considered nanowires, as opposed to nanoribbons constructed from a single layer of β-In2Se3.
Assuming that the two fullerene-like molecules prevent additional Se or In atoms from neighboring nanowires from binding to the existing nanowire during the heating experiment, the number of atoms in each nanoribbon/nanowire could be assumed to be constant before and after heating. With this knowledge, the two repeating units used to simulate the β-In2Se3 nanoribbon and the γ-In2Se3 nanowire were extended to 2D foils as shown in Figure 9a,b. A nanoribbon and nanowire, both containing 140 atoms (84 Se and 56 In), were constructed from these 2D films. These structures were fabricated to have the same appearance in projection as those shown above (Figures 7 and 8), while having different tailored defect locations to match the experimental AC-HRTEM images (Figure 5). For both the low-temperature β-In2Se3 nanowire and the high-temperature γ-In2Se3 nanowire, the dimensions of the simulated structures agree well with the experimental TEM images. The change in the volume of the nanowires after heating is further evidence that a phase change has occurred.
"Indium selenides have been shown to retain several desirable properties, such as ferroelectricity, tunable photoluminescence through temperature-controlled phase changes, and high electron mobility when confined to two dimensions (2D)", the scientists say. "More studies on phase control of sub-nm nanoribbons is needed for their application in tailored and versatile two-dimensional nanoelectronic devices in the future."
Resource: Cull, W. J., Skowron, S. T., Hayter, R., Stoppiello, C. T., Rance, G. A., Biskupek, J., Kudrynskyi, Z. R., Kovalyuk, Z. D., Allen, C. S., Slater, T. J. A., Kaiser, U., Patané, A., & Khlobystov, A. N. (2023). Subnanometer-wide indium selenide nanoribbons. ACS nano, 17(6), 6062-6072. https://doi.org/10.1021/acsnano.3c00670
-
Xu, K.; Yin, L.; Huang, Y.; Shifa, T. A.; Chu, J.; Wang, F.; Cheng, R.; Wang, Z.; He, J. Synthesis, properties and applications of 2D layered MIIIXVI (M = Ga, In; X = S, Se, Te) materials. Nanoscale. 2016, 8, 16802-16818.
-
Yang, Z.; Hao, J. Recent progress in 2D layered III-VI semiconductors and their hetero-structures for optoelectronic device applications. Adv. Mater. Technol. 2019, 4, 1900108.
-
Han, G.; Chen, Z. G.; Drennan, J.; Zou, J. Indium selenides: structural characteristics, synthesis and their thermoelectric performances. Small 2014, 10, 2747-2765.
-
Sun, Y.; Li, Y.; Li, T.; Biswas, K.; Patane,̀ A.; Zhang, L. New polymorphs of 2D indium selenide with enhanced electronic properties. Adv. Funct. Mater. 2020, 30, 2001920.
-
Popovic, S.; Tonejc, A.; Grzeta-Plenkovic, B.; Celustka, B.; Trojko, R. Revised and new crystal data for indium selenides. J. Appl. Crystallogr. 1979, 12, 416-420.
-
Van Landuyt, J.; Van Tendeloo, G.; Amelinckx, S. Phase Transitions in In2Se3 as studied by electron microscopy and electron diffraction. Phys. Status Solidi 1975, 30, 299-314.
-
Hao, Q.; Yi, H.; Su, H.; Wei, B.; Wang, Z.; Lao, Z.; Chai, Y.; Wang, Z.; Jin, C.; Dai, J.; Zhang, W. Phase identification and strong second harmonic generation in pure ε-InSe and its alloys. Nano Lett. 2019, 19, 2634-2640.
-
Yuan, K.; Yin, R.; Li, X.; Han, Y.; Wu, M.; Chen, S.; Liu, S.; Xu, X.; Watanabe, K.; Taniguchi, T.; Muller, D. A.; Shi, J.; Gao, P.; Wu, X.; Ye, Y.; Dai, L. Realization of quantum Hall effect in chemically synthesized InSe. Adv. Funct. Mater. 2019, 29, 1904032.
-
Wan, S.; Li, Y.; Li, W.; Mao, X.; Zhu, W.; Zeng, H. Room temperature ferroelectricity and a switchable diode effect in two-dimensional α-In2Se3 thin layers. Nanoscale 2018, 10, 14885-14892.
-
Sun, X.; Yu, B.; Ng, G.; Nguyen, T. D.; Meyyappan, M. III-VI compound semiconductor indium selenide (InxSey) nanowires: synthesis and characterization. Appl. Phys. Lett. 2006, 89, 233121.
-
Zhai, T.; Fang, X.; Liao, M.; Xu, X.; Li, L.; Liu, B.; Koide, Y.; Ma, Y.; Yao, J.; Bando, Y.; Golberg, D. Fabrication of high-quality In2Se3 nanowire arrays toward high-performance visible-light photodetectors. ACS Nano 2010, 4, 1596-1602.
-
Li, J.; Li, H.; Niu, X.; Wang, Z. Low-dimensional In2Se3 compounds: from material preparations to device applications. ACS Nano 2021, 15, 18683-18707.
-
Ho, P. H.; Chang, Y. R.; Chu, Y. C.; Li, M. K.; Tsai, C. A.; Wang, W. H.; Ho, C. H.; Chen, C. W.; Chiu, P. W. High-mobility InSe transistors: the role of surface oxides. ACS Nano 2017, 11, 7362-7370.
-
Xiang, R.; Inoue, T.; Zheng, Y.; Kumamoto, A.; Qian, Y.; Sato, Y.; Liu, M.; Tang, D.; Gokhale, D.; Guo, J.; Hisama, K.; Yotsumoto, S.; Ogamoto, T.; Arai, H.; Kobayashi, Y.; Zhang, H.; Hou, B.; Anisimov, A.; Maruyama, M.; Miyata, Y.; Okada, S.; Chiashi, S.; Li, Y.; Kong, J.; Kauppinen, E. I.; Ikuhara, Y.; Suenaga, K.; Maruyama, S. One-dimensional van der Waals heterostructures. Nanomaterials 2020, 367, 537-542.
-
Biskupek, J.; Skowron, S. T.; Stoppiello, C. T.; Rance, G. A.; Alom, S.; Fung, K. L. Y.; Whitby, R. J.; Levitt, M. H.; Ramasse, Q. M.; Kaiser, U.; Besley, E.; Khlobystov, A. N. Bond dissociation and reactivity of HF and H2O in a nano test tube. ACS Nano 2020, 14, 11178-11189.
-
Norman, L. T.; Biskupek, J.; Rance, G. A.; Stoppiello, C. T.; Kaiser, U.; Khlobystov, A. N. Synthesis of ultrathin rhenium disulfide nanoribbons using nano test tubes. Nano Res. 2022, 15, 1282-1287.
-
Faulques, E.; Kalashnyk, N.; Slade, C. A.; Sanchez, A. M.; Sloan, J.; Ivanov, V. G. Vibrational and electronic structures of tin selenide nanowires confined inside carbon nanotubes. Synth. Met. 2022, 284, 116968-116977.
-
Hu, Z.; Breeze, B.; Kashtiban, R. J.; Sloan, J.; Lloyd-Hughes, J. Zigzag HgTe nanowires modify the electron-phonon interaction in chirality-refined single-walled carbon nanotubes. ACS Nano 2022, 16, 6789-6800.
-
Goktas, N. I.; Wilson, P.; Ghukasyan, A.; Wagner, D.; McNamee, S.; LaPierre, R. R. Nanowires for energy: a review. Appl. Phys. Rev. 2018, 5, 041305-041330.
-
Bendall, J. S.; Ilie, A.; Welland, M. E.; Sloan, J.; Green, M. L. H. Thermal stability and reactivity of metal halide filled single-walled carbon nanotubes. J. Phys. Chem. B 2006, 110, 6569-6573.
-
Sagawa, R.; Togashi, W.; Akita, T.; Takai, Y. Molybdenum oxide crystals encapsulated inside carbon nanotubes by heat treatment in air. Surf. Interface Anal. 2012, 44, 797.
-
Stoppiello, C. T.; Biskupek, J.; Li, Z. Y.; Rance, G. A.; Botos, A.; Fogarty, R. M.; Bourne, R. A.; Yuan, J.; Lovelock, K. R. J.; Thompson, P.; Fay, M. W.; Kaiser, U.; Chamberlain, T. W.; Khlobystov, A. N. A one-pot-one-reactant synthesis of platinum compounds at the nanoscale. Nanoscale 2017, 9, 14385-14394.
-
Ebbesen, T. W. Wetting, filling and decorating carbon nanotubes. J. Phys. Chem. Solids 1996, 57, 951-955.
-
Xu, C.; Sloan, J.; Brown, G.; Bailey, S.; Williams, V. C.; Friedrichs, S.; Coleman, K. S.; Flahaut, E.; Hutchison, J. L.; Dunin-Borkowski, R. E.; Green, M. L. H. 1D lanthanide halide crystals inserted into single-walled carbon nanotubes. Chem. Commun. 2000, 1, 2427-2428.
-
Botos, A.; Biskupek, J.; Chamberlain, T. W.; Rance, G. A.; Stoppiello, C. T.; Sloan, J.; Liu, Z.; Suenaga, K.; Kaiser, U.; Khlobystov, A. N. Carbon nanotubes as electrically active nanoreactors for multi-step inorganic synthesis: sequential transformations of molecules to nanoclusters and nanoclusters to nanoribbons. J. Am. Chem. Soc. 2016, 138, 8175-8183.
-
Fujimori, T.; Dos Dantos, R. B.; Hayashi, T.; Endo, M.; Kaneko, K.; Tománek, D. Formation and properties of selenium double-helices inside double-wall carbon nanotubes: experiment and theory. ACS Nano 2013, 7, 5607-5613.
-
Huang, W.; Gan, L.; Li, H.; Ma, Y.; Zhai, T. Phase-engineered growth of ultrathin InSe flakes by chemical vapor deposition for high-efficiency second harmonic generation. Chem.-A Eur. J. 2018, 24, 15678-15684.
-
Bendall, J. S.; Ilie, A.; Welland, M. E.; Sloan, J.; Green, M. L. H. Thermal stability and reactivity of metal halide filled single-walled carbon nanotubes. J. Phys. Chem. B 2006, 110, 6569-6573.
-
Ballesteros, B.; Tobias, G.; Ward, M. A. H.; Green, M. L. H. Quantitative assessment of the amount of material encapsulated in filled carbon nanotubes. J. Phys. Chem. C 2009, 113, 2653-2656.
-
Liu, X.; Marangon, I.; Melinte, G.; Wilhelm, C.; Ménard-Moyon, C.; Pichon, B. P.; Ersen, O.; Aubertin, K.; Baaziz, W.; PhamHuu, C.; Bégin-Colin, S.; Bianco, A.Gazeau, F.; Bégin, D. Design of covalently functionalized carbon nanotubes filled with metal oxide nanoparticles for imaging, therapy, and magnetic manipulation. ACS Nano 2014, 8, 11290-11304.