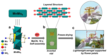
High-Resolution Imaging Validates 3D Network Formation in Novel Catalytic Polyoxometalate Aerogels
September 24, 2025 – Researchers from the Universities of Mainz, Ulm, and Jena (Germany) have achieved the first conversion of a polyoxometalate-based organogel into a catalytically active aerogel. The TRIS-functionalized Anderson polyoxometalate forms a gel within just 10 seconds when combined with ZnCl2 in acetonitrile. Electron microscopy reveals the aerogel's layered structure of thin fibrous sheets, while elemental mapping confirms a uniform distribution of all components. The new material shows promising catalytic activity for selective alcohol oxidation reactions, opening pathways to functional POM-based porous catalysts for energy and separation technologies.
Supramolecular gels represent a promising class of functional soft materials with unique structure and function. [1,2,3] Metallogels are particularly attractive because classical coordination chemistry between metal cations and suitable organic ligands can be exploited to design and tune gel properties and reactivities. [4,5] These linkages are further stabilized by non-covalent interactions such as hydrogen bonding, electrostatic forces, or hydrophobic interactions. [6,7] This versatility has enabled applications ranging from catalysis and sensing to optoelectronics and magnetism. [8,9,10,11] Metallogels can also be converted into technologically important aerogels [12] through freeze-drying, [13] an approach that removes the solvent from the gel structure while preserving its three-dimensional architecture. Aerogels are remarkable materials featuring high specific surface area, low density, and hierarchical pore structures. [14] These exceptional properties have driven their development in areas such as water treatment. [15] The use of diverse materials and their modification in aerogel construction has broadened applications from photocatalysis to the food industry. [16,17,18]
Molecular metal oxide anions, known as polyoxometalates (POMs), [19] have recently emerged as a new class of low molecular weight gelators for designing metallogels. POMs are versatile molecules with tunable structures and reactivities. In 2020, Centellas et al. developed dumbbell-type dimers linked via Co(II) or Co(III), constructed from Keggin and Dawson POMs functionalized with terpyridine ligands. The resulting metallogels exhibited intriguing solvent exchange properties, opening pathways to hydrogel formation. [20]
One prototype gel-forming POM is the organofunctionalized Anderson-Evans polyoxometalate [21] [XMo6O18{RC(CH2O)3}2]3- (where X = Mn, Fe, Co, and R = -NH2, -CH3, -CH2OH), which features two organic ligands arranged linearly on opposite sides of the cluster, tethered by tris-alkoxide groups (Fig. 1a). More recently, Casimiro et al. (2024) designed supramolecular metallogels by linking terpyridine-functionalized Anderson anions with Zn(II) or Co(II) cations. These hybrid gels exhibited intriguing properties including luminescence, birefringence, and spin-crossover behavior. [22] Until now, no conversion of pure POM-based metallogels into aerogels had been demonstrated.
In recent years, Anderson-Evans POMs have been extensively studied as catalysts for various oxidation reactions, demonstrating their potential to oxidize organic compounds such as thioanisoles and furan-based chemicals. [23,24] Building on these studies, the present work demonstrates the use of a POM-based aerogel for the oxidation of primary alcohols, paving the way for POM-based porous catalysts in organic transformations. The researchers developed a POM-based organogel that forms within 10 seconds by reacting ZnCl2 with TRIS-functionalized Anderson POM in acetonitrile solution. The resulting organogel can readily be converted into an aerogel through freeze-drying. The study provides comprehensive characterization of the aerogel and demonstrates its suitability for selective alcohol oxidation reactions using benzyl alcohol, furfuryl alcohol, and octanol as model substrates. Additionally, the team presents initial insights into the scope of gel formation, focusing on the roles of the POM, ZnCl2, and the solvent.
The POM-based organogel was prepared by combining acetonitrile solutions of ZnCl2 and the TRIS-functionalized Anderson anion (nBu4N)3[MnMo6O18{(OCH2)3CNH2}2]. [25] The vial inversion test [26] confirmed the stability and viscoelasticity of the gel (Fig 1b). Subsequently, the organogel was converted into aerogel (1) by freeze-drying (Fig. 1c).
To gain deeper structural insights into the gelation process, the researchers performed 1H NMR spectroscopic titrations, adding 0 to 2 molar equivalents of ZnCl2 to the MnMo6 solution in deuterated acetonitrile (Figure 2). Notably, the methylene protons of the TRIS-NH2 experience a significant paramagnetic shift due to the Mn(III) center of the Anderson POM. [25] Upon adding increasing amounts of ZnCl2, the team observed broadening and intensity decrease of all 1H NMR signals. The reduced molecular mobility resulting from gelation partially reintroduces anisotropic chemical shift and dipole-dipole couplings, affecting the spectral lines. Previous reports have shown similar line broadening of characteristic peaks when Keggin-type POMs triggered hydrogel formation with gamma-cyclodextrin. [27]
Thermogravimetric analysis (TGA) in air atmosphere revealed the thermal stability of compound 1. A significant weight loss of 27.6% (calculated: 28.3%) occurs between 200 and 385 °C, attributed to the loss of three nBu4N+ cations. A second weight loss of 18.4% between 410 and 555 °C corresponds to the simultaneous decomposition of the TRIS moieties [28] and volatilization of ZnCl2. [29,30] X-ray photoelectron spectroscopy revealed a spin-orbit coupled doublet of Mn 2p at binding energies of 640.9 and 652.7 eV, assigned to Mn 2p3/2 and Mn 2p1/2 of Mn(III), respectively (Figure 3b). [31] The Cl 2p spectrum (Fig. 3c) shows binding energies at 200.2 eV (Cl 2p1/2) and 198.5 eV (Cl 2p3/2), characteristic of Cl-Zn coordination. [32] The Zn 2p XP spectrum revealed two peaks at 1045.3 and 1022.4 eV, assigned to the Zn 2p1/2 and Zn 2p3/2 signals of Zn(II) species (Figure 3d). [33]
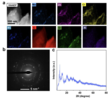
Scanning electron microscopy (SEM) revealed the morphology of compound 1 (Fig. 4a,b), showing a layered structure composed of stacked and agglomerated sheets. For further insights into the morphological and structural properties, the scientists employed transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM), and scanning-transmission electron microscopy (STEM) combined with energy dispersive X-ray spectroscopy (EDS). The images reveal that compound 1 consists of thin, elongated fibrous sheets that appear stacked (Fig 4c,d). HRTEM analysis (Fig. 4e,f) confirms the existence of both long fibrous and layered structures, likely resulting from the supramolecular linkage of Anderson anions.
High-angle annular dark-field scanning TEM (HAADF-STEM) imaging and corresponding EDS elemental mapping verified the uniform distribution of C, N, O, Cl, Mn, Zn, and Mo throughout compound 1 (Fig. 5a). Local selected area electron diffraction (SAED) analysis (Fig. 5b) indicates crystalline characteristics, with a calculated d-spacing of 4.83 nm-1 that agrees well with the d-spacing value of 4.40 nm-1 obtained from powder X-ray diffraction (pXRD) (Fig. 5c).
To assess the elemental distribution of Mn, Zn, Cl, and Mo in the bulk material, the researchers employed 2D micro X-ray fluorescence spectroscopy (2D-microXRF; resolution approximately 25 micrometers) on a small piece of compound 1 (Fig. 6). Homogeneous distribution of all four elements is evident from similar intensities across the bulk material, particularly within the selected (1 x 1) mm2 area indicated by the green square (Fig. 6a). This becomes more apparent when examining the intensity histograms generated for this area (Fig. 6b), which display a normal distribution of intensities without extreme values.
POMs have been extensively investigated as catalysts for various organic oxidation reactions. [34,35,36] Anderson-type POMs in particular have proven highly efficient for alcohol oxidation. [37,38,39] Following the reaction, the catalyst was separated via centrifugation, and product analysis was performed using quantitative 1H NMR spectroscopy. The oxidation data for benzyl alcohol demonstrate that compound 1 achieves significantly higher benzaldehyde yields compared to the blank sample (Fig. 7a). Similarly, substantially higher furfural and octanal yields were observed with compound 1 as catalyst (Fig. 7b) over time. These results indicate that compound 1 exhibits higher activity for benzyl alcohol oxidation than for furfuryl alcohol or octanol oxidation, possibly related to the more complex molecular structures of furfuryl alcohol and octanol.
The study demonstrates a facile approach to synthesize compound 1 based on TRIS-functionalized Anderson polyoxometalate anions as low molecular weight gelators when crosslinked with ZnCl2 in acetonitrile. The resulting gel showed promising initial alcohol oxidation reactivity for benzyl alcohol and the bio-based furfuryl alcohol. The findings provide initial insights into the individual roles of POM anion, metal salt, and solvent, offering guidance for the future development of this new class of POM-based porous catalysts.
Resource:
Sachdeva, G., Maloul, S., Zolg, J., Müller, R., Mondeshki, M., Ebrahimi, E., Abid, D., Chala, S. A., Neumann, C., Turchanin, A., Biskupek, J., Kaiser, U., Leopold, K., Streb, C. (2025).
Polyoxometalate Aerogels Formed by Organofunctionalized Anderson Polyoxometalates as Low Molecular Weight Gelators.
Adv. Mater. Interfaces 2025, e00597.
https://doi.org/10.1002/admi.202500597
Christoff-Tempesta, A., et al. (2018). Beyond covalent crosslinks: applications of supramolecular gels. Gels 4, 40. https://doi.org/10.3390/gels4020040
Smith, D. (2024). Supramolecular gels - a panorama of low-molecular-weight gelators from ancient origins to next-generation technologies. Soft Matter 20, 10. https://doi.org/10.1039/D3SM01301D
Sangeetha, N. M. & Maitra, U. (2005). Supramolecular gels: Functions and uses. Chem. Soc. Rev. 34, 821. https://doi.org/10.1039/b417081b
Picci, G., et al. (2023). Metal-based gels: Synthesis, properties, and applications. Coord. Chem. Rev. 492, 215225. https://doi.org/10.1016/j.ccr.2023.215225
Fortunato, A. & Mba, M. (2021). Metal cation triggered peptide hydrogels and their application in food freshness monitoring and dye adsorption. Gels 7, 85. https://doi.org/10.3390/gels7030085
Steed, J. W. (2010). Anion-tuned supramolecular gels: a natural evolution from urea supramolecular chemistry. Chem. Soc. Rev. 39, 3686. https://doi.org/10.1039/B926219A
Zhang, J. & Su, C. Y. (2013). Metal-organic gels: From discrete metallogelators to coordination polymers. Coord. Chem. Rev. 257, 1373. https://doi.org/10.1016/j.ccr.2013.01.005
Dhibar, S., et al. (2020). Triethylenetetramine-based semiconducting Fe (III) metallogel: Effective catalyst for Aryl-S coupling. ACS Omega 5, 2680. https://doi.org/10.1021/acsomega.9b03194
Grondin, O. et al. (2010). Multifunctional gels from polymeric spin-crossover metallo-gelators. Langmuir 26, 5184. https://doi.org/10.1021/la903653d
Roy, A. (2024). An innovative semiconducting Ni(II)-metallogel based robust random access memory (RRAM) device for advanced flexible electronics applications. Sci. Rep. 14, 31619. https://doi.org/10.1038/s41598-024-79358-3
Dhibar, S. et al. (2023). A transparent self-healable multistimuli-responsive novel supramolecular Co(II)-metallogel derived from adipic acid: Effective hole transport layer for polymer solar cells. Mol. Liq. 370, 121020. https://doi.org/10.1016/j.molliq.2022.121020
Sonu, S., et al. (2023). Multifunctional aerogels: A comprehensive review on types, synthesis and applications of aerogels. J. Sol-Gel Sci. Technol. 105, 324. https://doi.org/10.1007/s10971-022-06026-1
Walker, R. C., et al. (2021). Zirconia aerogels for thermal management: Review of synthesis, processing, and properties information architecture. Adv. Col. Int. Sci. 295, 102464. https://doi.org/10.1016/j.cis.2021.102464
Smirnova, I. & Gurikov, P. (2018). Aerogel production: Current status, research directions, and future opportunities. J. Supercrit. Fluids 134, 228. https://doi.org/10.1016/j.supflu.2017.12.037
Ganesamoorthy, R. (2021). Aerogels for water treatment: A review. J. Clean Prod. 329, 129713. https://doi.org/10.1016/j.jclepro.2021.129713
Liu, Z., et al. (2023). Recent advances in stimuli-responsive metallogels. Molecules 28, 2274. https://doi.org/10.3390/molecules28052274
Khan, N. R. (2024). Exploring the versatility of aerogels: Broad applications in biomedical engineering, astronautics, energy storage, biosensing, and current progress. Heliyon 10, 23102. https://doi.org/10.1016/j.heliyon.2023.e23102
Scognamiglio, P. L., et al. (2025). Metallogels as supramolecular platforms for biomedical applications: A review. Processes 13, 3671.
Liu, Q. & Wang, X. (2020). Polyoxometalate clusters: Sub-nanometer building blocks for construction of advanced materials. Matter 2, 816. https://doi.org/10.1016/j.matt.2020.01.020
Centellas, M. S., et al. (2020). Exploring the self-assembly of dumbbell-shaped polyoxometalate hybrids, from molecular building units to nanostructured soft materials. Chem. Sci. 11, 11072. https://doi.org/10.1039/D0SC03243C
Blazevic, A. & Rompel, A. (2016). The Anderson-Evans polyoxometalate: From inorganic building blocks via hybrid organic-inorganic structures to tomorrows 'Bio-POM'. Coord. Chem. Rev. 307, 42. https://doi.org/10.1016/j.ccr.2015.07.001
Casimiro, L., et al. (2024). Multifunctional supramolecular gels with strong mechanical properties formed by self-assembly of polyoxometalate-based coordination polymers. JACS Au 4, 4948. https://doi.org/10.1021/jacsau.4c00981
Wang, Y., et al. (2018). Ratio-controlled precursors of Anderson-Evans polyoxometalates: Synthesis, structural transformation, and magnetic and catalytic properties of a series of triol ligand-decorated {M2Mo6} Clusters (M = Cu2+, Co2+, Ni2+, Zn2+). Inorg. Chem. 57, 3731. https://doi.org/10.1021/acs.inorgchem.7b02996
Raabe, J. C., et al. (2024). Synthesis and characterization of V substituted Anderson-type telluromolybdates and tungstates for catalytic oxidation of furan derivatives to formic and maleic acid. Catal. Today 441, 114899. https://doi.org/10.1016/j.cattod.2024.114899
Marcoux, P. B., et al. (2003). Developing remote metal binding sites in heteropolymolybdates. Eur. J. Inorg. Chem. 2003, 2406. https://doi.org/10.1002/ejic.200200677
Raghavan, S. R. & Cipriano, B. H. (2005). Gel formation: Phase diagrams using tabletop rheology and calorimetry. Springer, Dordrecht (Eds.: R.G. Weiss, P. Terech), pp. 241-252. https://doi.org/10.1007/1-4020-3689-2_9
Khlifi, S., et al. (2024). Switchable redox and thermo-responsive supramolecular polymers based on cyclodextrin-polyoxometalate tandem. Chem. - Eur. J. 30, 202303815. https://doi.org/10.1002/chem.202303815
Mahvash, S. et al. (2023). Anderson-type manganese polyoxomolybdate hybrid nanocomposite for boosting drug delivery against breast cancer. J. Drug Deliv. Sci. Technol. 87, 104778. https://doi.org/10.1016/j.jddst.2023.104778
Jones, F. (2013). Thermal stability of zinc compounds. Energy and Fuels 27, 5663. https://doi.org/10.1021/ef400505u
Tancredi, N., et al. (2017). Thermal studies of wood impregnated with ZnCl2. Eur. J. Wood & Wood Prod. 75, 633.
Peng, C. (2020). Anderson-type polyoxometalate-based hybrid with high flame retardant efficiency for the preparation of multifunctional epoxy resin nanocomposites. Compos. B Eng. 186, 107780. https://doi.org/10.1016/j.compositesb.2019.107780
Hoang, D., et al. (2023). Vanillin: An effective additive to improve the longevity of Zn metal anode in a 30 m ZnCl2 electrolyte. Adv. Energy Mater. 13, 1. https://doi.org/10.1002/aenm.202301712
Schmidt, S., et al. (2013). Preparation and characterization of neat and ZnCl2 modified zeolites and alumina for methyl chloride synthesis. Appl. Catal. A Gen. 468, 120. https://doi.org/10.1016/j.apcata.2013.08.039
Hill, C. L., et al. (1995). Homogeneous catalysis by transition metal oxygen anion clusters. Coord. Chem. Rev. 143, 407. https://doi.org/10.1016/0010-8545(95)01141-B
Wei, Z., et al. (2022). Recent advances of Anderson-type polyoxometalates as catalysts largely for oxidative transformations of organic molecules. Molecules 27, 5212. https://doi.org/10.3390/molecules27165212
Barats, D. & Neumann, R. (2010). Aerobic oxidation of primary aliphatic alcohols to aldehydes catalyzed by a palladium (II) polyoxometalate catalyst. Adv. Synth. Catal. 352, 293. https://doi.org/10.1002/adsc.200900663
Khenkin, A. M., et al. (2003). Preparation and characterization of new ruthenium and osmium containing polyoxometalates, [M(DMSO)3Mo7O24]4- (M = Ru(II), Os(II)), and their use as catalysts for the aerobic oxidation of alcohols. Inorg. Chem. 42, 3331. https://doi.org/10.1021/ic026003p
Zhang, M., et al. (2018). Highly practical and efficient preparation of aldehydes and ketones from aerobic oxidation of alcohols with an inorganic-ligand supported iodine catalyst. Chem. Commun. 54, 10164. https://doi.org/10.1039/c8cc03722a
Wei, Z., et al. (2019). Highly efficient and practical aerobic oxidation of alcohols by inorganic-ligand supported copper catalysis. Green Chem. 21, 4069. https://doi.org/10.1039/C9GC01248F