Atom-by-Atom Transformation of Few-Layer MnPS3
March 14, 2023 - Manganese phosphorus trisulfide (MnPS3) is a particularly interesting material in the context of magnetism in a system with reduced dimensionality. A team of researchers from the Universities of Ulm and the Helmholtz-Centre Dresden-Rossendorf in Germany has presented an experimental and theoretical study on modifying the properties of freestanding quasi-two-dimensional (2D) MnPS3 by local structural transformations via electron irradiation and thermal annealing that may provide opportunities to build new devices for starge of quantum information.
Material design is an attractive idea that is very difficult to implement in practice. Combining the best of different ingredients to create the ultimate material is a task[1] for which we need an optimal tool that can change the material structure at the atomic level.[2] The ability to characterize and change materials at the atomic level is as important for progress in nanotechnology[3,4] as precise control over their growth. Aberration-corrected (AC) high-resolution (HR) in situ transmission electron microscopy (TEM),[5,6] along with scanning transmission electron microscopy (STEM),[7] have been shown to be especially useful as they allow to obtain information about the atomic rearrangement in the sample due to electron irradiation in real time.[2,8] Using (S)TEM techniques, depending on the choice of electron beam size, structural changes can be made locally down to the atomic level[9] as a result of the interaction of the highly accelerated electrons with the sample.[10,11]
The modification of ultrathin nanosheets has recently attracted considerable attention because the structure and thus the properties of two-dimensional (2D) materials can be more easily altered (compared to bulk systems) due to their geometry. In particular, the crystallographic, electronic, and magnetic properties of 2D materials[12] can be tuned by creating single-[13] and extended defects[14-16] and by adsorbing atoms.[17] In addition, (S)TEM can be used to effect spatially confined phase transformations in crystalline materials with few layers or monolayers, thereby significantly changing their local properties. (S)TEM studies have been performed on various low-dimensional materials. Controllable structural transformations in transition metal dichalcogenides (TMDs),[13,18-20] alumina,[21] silica,[21] and nanowires[22,23] have been reported. The advantage of electron beam-mediated modification over other conventional methods (e.g., heating,[24] electrostatic gating,[25] strain engineering[26]) for inducing 2D phase transformations is the real-time control of the transformation process down to the atomic scale.[19,22] Thus, (S)TEM is an excellent tool for locally controlling the properties of 2D materials.
Within the class of 2D materials, transition metal phosphorus trisulfides (TMPTs) have recently come into focus of researchers. For scientists, manganese phosphorus trisulfide (MnPS3) has received the most attention. MnPS3 crystallizes in the monoclinic space group C2/m,[27] in which the magnetic Mn ions2+ are coordinated octahedrally to six S atoms with a dumbbell-shaped structure [PS]264- occupied by P-P groups in the centers of the hexagons.[28] MnPS3 has inherent magnetic properties,[29] which persist even when the material is thinned down to a few layers,[30] with a band gap of about 3 eV,[31] making it an ideal candidate for multifunctional electronics/spintronics[32] and battery[33] applications. More interestingly, the intrinsic properties of MnPS3 can be significantly manipulated. For example, pressure-controlled compression has been shown to significantly alter the magnetic properties and force an insulator-to-metal transition.[34,35] Furthermore, with suitable intercalations, there is a reduction in intralayer coupling and a change in the magnetic properties of MnPS3.[36,37] In addition, it has already been shown that MnS-type systems are promising candidates for various applications. For example, α-MnS has been proposed as an anode material for postlithium batteries[38,39] and is an interesting candidate for future electronic devices, e.g., in field-effect transistors.[40] The authors of the new study had found earlier that TMPTs with few layers lose predominantly sulfur due to the interaction with the incident electrons, which in turn leads to strong changes in the original material.[41] Now they turned to the investigation of the properties of free-standing MnPS3/MnS systems due to electron beam-induced phase transformations and annealing.
Analysis of the structural modification
Figure 2 (a1- 3) shows an image sequence of 80 kV Cc/Cs corrected HRTEM of MnPS3 under the electron beam with a constant dose rate of 0.5 × 106 e−/(snm2). Figure 2 (a1) shows the original material in the normal incidence direction [103] (Figure 1) of MnPS3 , with an enlarged region on the right. After irradiation, changes in the material can be seen as shown in Figure 2 (a2-3): Islands with an altered structure grow continuously within the basic matrix. As shown in our previous study, primary removal of sulfur (S) and phosphorus (P) atoms is favored by 80 keV electrons via direct electron-nucleus collisions.[41] This leads to S deficiency in electron beam exposed regions of MnPS3 and therefore can lead to the growth of new structures.
Extensive studies (TEM experiments and calculations) with a variety of materials have shown that atoms in layers facing the electron beam are significantly less affected by direct electron-nuclear collisions during electron beam sputtering than the beam exit surface (in this case, the lower layer).[41] In particular, when considering a single layer, the displacement thresholds for chalcogen atoms facing the beam (in the upper chalcogen layer) were found to be higher than for the lower chalcogen layer.[8,42] Assuming that there are other layers on top of a single layer (few-layer), it can be assumed that the outer layers of the few-layer system are primarily affected by the electron beam (or most likely the lower layer, see inset in Figure 2b). To illustrate the presumed transformation process in MnPS3, an experimental and two corresponding FFT mask filtered images are shown in Figure 2c. When the MnPS3 atomic lattice is filtered with the FFT mask, the structure extends over the entire sample area. In contrast, the new structure is only present in the areas outlined in red. From this, the scientists concluded that the new phase primarily spreads laterally (in-plane), while intact MnPS3 layers are present at the top or bottom. A modeled bilayer scheme below visualizes the new structure emerging (red), embedded in an isolated MnPS3 layer (gray).
3D-ED was used to determine the lattice parameters (unit cell) of the newly formed phases.[43] MnPS3 with few layers was irradiated until additional Bragg reflections of the new phases were clearly visible in the ED data (see Figure 3a,b). Two different new phases (labeled as phase 1 and 2) can be seen; the reflections of phase 1 have a higher intensity and correspond to the observed embedded phase from Figure 2. The irradiated crystal was tilted in the TEM in a range of ±60° with a tilt step of 1°. A diffraction pattern was recorded at each tilt position of the crystal. A tilt series of the patterns was reconstructed in a reciprocal 3D space. The diffraction volume[43] in Figure 3c shows an overlay of two distinct crystalline lattices - the MnPS3 host (blue) and the new phase 1 (red).
Analysis of the ED pattern at perpendicular incidence in Figure 3a,b revealed an additional set of weak diffraction spots aligned with the other two gratings (MnPS3 and cubic α-MnS1-xPx) and belonging to the unidentified phase 2. These reflections occurred at later stages of structural transformation, and the reflections appeared as small arcs; sometimes parts of diffraction rings were also visible. We attribute this to a partial loss of the phase 2 orientation relationship with the host MnPS3. The search for a suitable phase candidate yielded the hexagonal γ-MnS1-xPx (see Figure 3d), with a wurtzite structure.[44] The structural characterization of this phase was complicated by the presence of the other two phases. Nevertheless, the reflectance positions agree well with the observed data. For example, the peak at 1.98 Å (in Figure 3b) is consistent with the 21̅1̅0 γ-MnS1-xPx reflection. Moreover, ultrathin patches of phase 2 at a later transformation stage could also be found in the experimental HRTEM images.
The experiments indicate that at the beginning of the irradiation process, the electron beam causes the transformation of the host material into cubic α-MnS1-xPx oriented with the [111] direction parallel to the normal incidence [103] of the MnPS3 host matrix. With increasing imaging time (i.e., larger total accumulated dose), freestanding patches of the newly formed phases could be imaged after the host material was completely degraded. Figure 4a shows an experimental HRTEM image of a freshly grown isolated α-MnS1-xPx crystal. A magnified section of the image is shown in the red frame. Superposition of the simulated image with the atomic model of the cubic α-MnS1-xPx phase oriented along the [111] direction highlights the atomic column contrast. The lattice parameters are consistent with those of the found α-MnS1-xPx phase in the 3D ED (see Figure 3). At this transformation stage, different orientations of the α-MnS1-xPx occur and can also be imaged as free-standing structures. Figure 4b shows the HRTEM image of a spot along the [001] direction of α-MnS1-xPx, with a magnified portion in the green outlined area. The experimental image agrees well with the simulated HRTEM image for this orientation. Figure 4c shows a region of the γ-MnS1-xPx in the [0001] direction; a magnified image of this region is shown in blue. The observed contrast agrees well with the corresponding image simulations of γ-MnS1-xPx [0001].
The energetics of the α-MnS1-xPx and γ-MnS1-xPx phases formed at room temperature were studied using first-principles calculations. To analyze the stability of such compounds, the convex shell was constructed based on the enthalpy of formation per atom. The use of a convex hull is a standard approach in materials science to evaluate the relative stability of materials with different stoichiometries. In the case of α-[111] MnS1-xPx , several mixed compounds (e.g., for x = 0, 0.625, 1) are located on the convex hull, suggesting that these materials should be at least metastable. As with other unstable compounds, the energy differences with respect to the convex hull are in the meV range, so that at room temperature the mixing entropy is[45] per atom can stabilize the compounds (see Figure 4), with the Gibbs free energy difference ΔG = ΔH - TSmix to the convex hull plotted on the right. Compared to the [111] direction, the MnS1-xPx sheets with the surface oriented in the [001] direction show higher stability over a wide range of low P concentrations. In the case of the γ-phase, stabilization of the mixed MnS1-xPx compound in the [0001] direction requires a higher amount of phosphorus than in the α-phase.
Analysis of the elemental composition
To analyze the elemental composition of the annealed structure, STEM HAADF images and EDX elemental maps (80 kV) were acquired from different sample positions (Figure 5a) annealed at a temperature of 600 °C. The HAADF images show the altered structure, and the elemental maps of Mn and S demonstrate the presence of these elements. Figure 5b shows a detailed EDX spectrum of the annealed sample, and the observed peaks are labeled with the corresponding elemental edges. In addition, the corresponding atomic fractions and defects are shown in a table in Figure 5b. Mainly Mn and S are seen, as well as small amounts of C, O, and residual P. These results indicate that additional contamination by carbon as well as residual oxidized Mn, P and S is present.
To determine the valence, core loss EELS of the Mn-L2,3 edge is performed and shown in Figure 5c. The ratio of the white line[46] from L3 to L2 gives a value of 4.50, indicating that the structure contains mainly Mn(2+).[41,46] To determine the valence, core loss EELS of the Mn-L2,3 edge was performed and shown in Figure 5c. The ratio of the white line71 of L3 to L2 gives a value of 4.50, suggesting that the structure contains mainly Mn(2+).[41,46] Electron diffraction experiments were also performed on the annealed sample in selected areas; an azimuthally integrated pattern (adjusted for elliptical distortions) is shown in Figure 5d. A simulated powder diffraction pattern of alabandite - face-centered cubic Mn +S22- - is shown in red. The simulated and experimental patterns agree well, indicating the formation of alabandite due to annealing of pure MnPS3. An additional peak at 0.45 Å−1 corresponds to 2.2 Å and is probably related to graphitic impurities formed on the surface (the presence of carbon is also detected in EDX elemental quantification (see the table in Figure 5b).
To verify the formation of alabandite, the annealed structure was imaged in Cc/Cs-corrected HRTEM at 80 kV. A typical crystalline grain is shown in Figure 5e, and its magnified region is shown in Figure 5f. A cubic lattice aligned along the [100] direction can be clearly seen. The distances in the x and y directions between the atomic columns in Figure 5f are 2.60 and 2.58 Å, respectively, which agrees well with the expected value of 2.62 Å[43] for the alabandite structure projected in [100]. The FFT of the image in Figure 5e is shown in Figure 5g, and the measured reciprocal distances (0.38, 0.54, 0.77 Å−1 ) of the observed reflections agree well with the azimuthally integrated distances in Figure 5d).
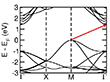
We further investigated the electronic and magnetic properties of the new MnS polymorphs using DFT calculations. The energetically preferred magnetic order was determined for single- and bilayer systems (see Figure 6). The stability of the facets was investigated for both ferromagnetic (FM) and antiferromagnetic (AFM) orientations of the spins in the Mn layers. In the case of the α-MnS phase, the [001] facet is more stable in the AFM state than in the FM state, while the [111] facet prefers to remain in the FM state. However, in the case of the γ-MnS phase, the thickness of the slab affects the magnetic character of the system by changing the order from ferromagnetic to antiferromagnetic. Our results show that the electronic structure of the bulk MnS is significantly changed in the new polymorphs. For the bulk material, DFT+U calculations reveal a band gap of 1.43 eV due to a transition between the valence band maximum (VBM) at the M point and the conduction band minimum (CBM) at the Γ point (see Figure 7 and Table S1). In the case of the plates, the material can be either semiconducting or metallic depending on the orientation and thickness of the facets. In the limiting case of the MnS monolayer, the α-[001] and γ-[0001] phases are semiconductors with band gaps of 0.03 and 0.23 eV, while the α-[111] monolayer has a metallic character. Since PBE generally underestimates the electronic band gaps (see, e.g., Ref. [47] and references therein), we further calculated the band gaps using more advanced approaches such as DFT+U and the Heyd-Scuseria-Ernzerhof (HSE) hybrid functional (Table 1).
"In situ Cc/Cs-corrected HRTEM experiments provided again insights into the annealing and electron beam induced structural changes of MnPS3," the scientists say. "These phase transformations can be controlled locally by the size of the electron beam as well as by the total electron dose applied, and simultaneously mapped on an atomic scale. For the MnS structures produced in this process, ab initio calculations showed that their electronic and magnetic properties strongly depend on the in-plane orientation and thickness of the crystallites. Appropriate dose rates enable controlled growth of α-MnS-type phases embedded in the host system, with crystals along the direction of the parent MnPS3. Another way to convert MnPS3 to pure α-MnS was demonstrated by in situ annealing under vacuum conditions."
The DFT calculations of the scientists showed that the pure MnS phases can exhibit different magnetic and electronic properties depending on the orientation and thickness of the facets. In particular, an α-[001]-MnS monolayer is an antiferromagnetic semiconductor with an indirect band gap, while a γ-[0001]-MnS monolayer is a ferromagnetic semiconductor with a direct band gap. We have also shown that the confinement effects in the γ-[0001]-MnS facets are strong, leading to a transition from the direct to the indirect band gap. In addition, it was found that alloying with phosphorus (i.e., MnS1-xPx, 0 ≤ x < 1) is a powerful technique for tailoring the electronic and optical properties of the MnS facets. Finally, we demonstrated that the mixed MnS1-xPx phases of both α- and γ-type can be stable for certain phosphorus concentrations.
Resource: Storm, A., Köster, J., Ghorbani-Asl, M., Kretschmer, S., Gorelik, T. E., Kinyanjui, M. K., Krasheninnikov, A. V., & Kaiser, U. (2023). Electron-Beam-and Thermal-Annealing-Induced Structural Transformations in Few-Layer MnPS3. ACS Nano, 17(5), 4250-4260. https://doi.org/10.1021/acsnano.2c05895
-
Novoselov, K. S.; Mishchenko, A.; Carvalho, O. A.; & Castro Neto, A. H. 2D materials and van der Waals heterostructures. Science 2016, 353, 461.
-
Zhao, X.; Kotakoski, J.; Meyer, J. C.; Sutter, E.; Sutter, P.; Krasheninnikov, A. V.; Kaiser, U.; & Zhou, W. Engineering and modifying two-dimensional materials by electron beams. Mrs Bulletin 2017, 42, 667-676.
-
Watanabe, T. Grain boundary engineering: historical perspective and future prospects. J. Mater. Sci. 2011, 46, 4095-4115.
-
Zubko, P.; Gariglio, S.; Gabay, M.; Ghosez, P.; Triscone, J. M. Interface physics in complex oxide heterostructures. Annu. Rev. Condens. Matter Phys. 2011, 2, 141-165.
-
Wang, L.; Xu, Z.; Wang, W.; Bai, X. Atomic mechanism of dynamic electrochemical lithiation processes of MoS2 nanosheets. J. Am. Chem. Soc. 2014, 136, 6693-6697.
-
Kühne, M.; Börrnert, F.; Fecher, S.; Ghorbani-Asl, M.; Biskupek, J.; Samuelis, D.; Krasheninnikov, A. V.; Kaiser, U.; Smet, J. H. Reversible superdense ordering of lithium between two graphene sheets. Nature 2018, 564, 234-239.
-
Liu, C.; Malladi, S. K.; Xu, Q.; Chen, J.; Tichelaar, F. D.; Zhuge, X.; Zandbergen, H. W. In-situ STEM imaging of growth and phase change of individual CuAlX precipitates in Al alloy. Sci. Rep. 2017, 7, 3-10.
-
Lehnert, T.; Lehtinen, O.; Algara-Siller, G.; Kaiser, U. Electron radiation damage mechanisms in 2D MoSe2 . Appl. Phys. Lett. 2017, 110, no. 033106.
-
Susi, T.; Meyer, J. C.; Kotakoski, J. Manipulating low-dimensional materials down to the level of single atoms with electron irradiation. Ultramicroscopy 2017, 180, 163-172.
-
Susi, T.; Kotakoski, J.; Arenal, R.; Kurasch, S.; Jiang, H.; Skakalova, V.; Stephan, O.; Krasheninnikov, A. V.; Kauppinen, E. I.; Kaiser, U.; Meyer, J. C. Atomistic description of electron beam damage in nitrogen-doped graphene and single-walled carbon nanotubes. ACS Nano 2013, 7, 7436.
-
Meyer, J. C.; Eder, F.; Kurasch, S.; Skakalova, V.; Kotakoski, J.; Park, H. J.; Roth, S.; Chuvilin, A.; Eyhusen, S.; Benner, G.; Krasheninnikov, A. V.; Kaiser, U. Accurate measurement of electron beam induced displacement cross sections for single-layer graphene. Phys. Rev. Lett. 2012, 108, 196102.
-
Wang, Q. H.; Bedoya-Pinto, A.; Blei, M.; Dismukes, A. H.; Hamo, A.; Jenkins, S.; Koperski, M.; Liu, Y.; Sun, Q.-C.; Telford, E. J.; Kim, H. H., Augustin, M.; Vool, U.; Yin, J.-.X.; Li, L. H.; Falin, A.; Dean, C. R.; Casanova, F.; Evans, R. F. L.; Chshiev, M.; Mishchenko, A.; Petrovic, C.; He, R.; Zhao, L.; Tsen, A. W.; Gerardot, B. D., Brotons-Gisbert, M.; Guguchia, Z.; Roy, X.; Tongay, S.; Wang, Z.; Hasan, M. Z.; Wrachtrup, J.; Yacoby, A.; Fert, A.; Parkin, S.; Novoselov, K. S.; Dai, P.; Balicas, L.; & Santos, E. J. The magnetic genome of two-dimensional van der Waals materials. ACS nano 2022, 16, 6960-7079.
-
Lin, Y. C.; Björkman, T.; Komsa, H. P.; Teng, P. Y.; Yeh, C. H.; Huang, F. S.; Lin, K. H.; Jadczak, J.; Huang, Y. S.; Chiu, P. W.; Krasheninnikov, A. V.; Suenaga, K. Three-fold rotational defects in two-dimensional transition metal dichalcogenides. Nat. Commun. 2015, 6, 6736.
-
Komsa, H. P.; Kurasch, S.; Lehtinen, O.; Kaiser, U.; Krasheninnikov, A. V. From point to extended defects in two-dimensional MoS2 : evolution of atomic structure under electron irradiation. Phys. Rev. B 2013, 88, no. 035301.
-
Wang, S.; Lee, G. Do; Lee, S.; Yoon, E.; Warner, J. H. Detailed atomic reconstruction of extended line defects in monolayer MoS2 . ACS Nano 2016, 10, 5419-5430.
-
Lehnert, T.; Ghorbani-Asl, M.; Köster, J.; Lee, Z.; Krasheninnikov, A. V.; Kaiser, U. Electron-beam-driven structure evolution of single-layer MoTe2 for quantum devices. ACS Appl. Nano Mater. 2019, 2, 3262-3270.
-
Meyer, J. C.; Kurasch, S.; Park, H. J.; Skakalova, V.; Künzel, D.; Groß, A.; Chuvilin, A.; Algara-Siller, G.; Roth, S.; Iwasaki, T.; Starke, U.; Smet, J. H.; Kaiser, U. Experimental analysis of charge redistribution due to chemical bonding by high-resolution transmission electron microscopy. Nat. Mater. 2011, 10, 209-215.
-
Lin, Y. C.; Dumcenco, D. O.; Huang, Y. S.; Suenaga, K. Atomic mechanism of the semiconducting-to-metallic phase transition in single-layered MoS2 . Nat. Nanotechnol. 2014, 9, 391-396.
-
Sutter, E.; Huang, Y.; Komsa, H. P.; Ghorbani-Asl, M.; Krasheninnikov, A. V.; Sutter, P. Electron-beam induced transformations of layered tin dichalcogenides. Nano Lett. 2016, 16, 4410-4416.
-
Lin, Y.; Komsa, H.; Yeh, C.; Björkman, T.; Liang, Z.-Y.; Ho, C.-H.; Huang, Y.-S.; Chiu, P.-W.; Krasheninnikov, A. V.; Suenaga, K. Single-layer ReS2 : Two-dimensional semiconductor with tunable in-plane anisotropy. ACS Nano 2015, 9, 11249-11257.
-
Chen, C. L.; Arakawa, K.; Lee, J. G.; Mori, H. Electron-irradiation-induced phase transformation in alumina. Scr. Mater. 2010, 63, 1013-1016.
-
Björkman, T.; Kurasch, S.; Lehtinen, O.; Kotakoski, J.; Yazyev, O. V.; Srivastava, A.; Skakalova, V.; Smet, J. H.; Kaiser, U.; Krasheninnikov, A. V. Defects in bilayer silica and graphene: common trends in diverse hexagonal two-dimensional systems. Sci. Rep. 2013, 3, 3482.
-
Zhang, Z.; Liu, N.; Li, L.; Su, J.; Chen, P. P.; Lu, W.; Gao, Y.; Zou, J. In situ TEM observation of crystal structure transformation in InAs nanowires on atomic scale. Nano Lett. 2018, 18, 6597-6603.
-
Zhang, H.; Wang, W.; Xu, T.; Xu, F.; Sun, L. Phase transformation at controlled locations in nanowires by in situ electron irradiation. Nano Res. 2020, 13, 1912-1919.
-
Brown, B. E. The crystal structures of WTe2 and high-temperature MoTe2 . Acta Crystallogr. 1966, 20, 268-274.
-
Cho, S.; Kim, S.; Kim, J. H.; Zhao, J.; Seok, J.; Keum, D. H.; Baik, J.; Choe, D.; Chang, K. J.; Suenaga, K.; Kim, S. W.; Lee, Y. H.; Yang, H. Phase patterning for ohmic homojunction contact in MoTe2 . Science 2015, 349, 625-628.
-
Meng, L.; Ma, Y.; Si, K.; Xu, S.; Wang, J.; Gong, Y. Recent advances of phase engineering in group VI transition metal dichalcogenides. Tungsten 2019, 1, 46-58.
-
Brec, R. Review on structural and chemical properties of transition metal phosphorous trisulfides MPS3 . Solid State Ionics 1986, 22, 3-30.
-
Lee, S.; Choi, K. Y.; Lee, S.; Park, B. H.; Park, J. G. Tunneling transport of mono- and few-layers magnetic van der Waals MnPS3 . APL Mater. 2016, 4, no. 086108.
-
Chittari, B. L.; Park, Y.; Lee, D.; Han, M.; Macdonald, A. H.; Hwang, E.; Jung, J. Electronic and magnetic properties of single-layer MPX3 metal phosphorous trichalcogenides. Phys. Rev. B 2016, 94, 184428.
-
Long, G.; Henck, H.; Gibertini, M.; Dumcenco, D.; Wang, Z.; Taniguchi, T.; Watanabe, K.; Giannini, E.; Morpurgo, A. F.. Persistence of magnetism in atomically thin MnPS3 crystals. Nano Lett. 2020, 20, 2452-2459.
-
Du, K. Z.; Wang, X. Z.; Liu, Y.; Hu, P.; Utama, M. I. B.; Gan, C. K.; Xiong, Q.; Kloc, C. Weak van der Waals stacking, wide-range band gap, and Raman study on ultrathin layers of metal phosphorus trichalcogenides. ACS Nano 2016, 10, 1738-1743.
-
Kumar, R.; Jenjeti, R. N.; Austeria, M. P.; Sampath, S. Bulk and few-layer MnPS3 : a new candidate for field effect transistors and UV photodetectors. J. Mater. Chem. C 2019, 7, 324-329.
-
Glass, D. E.; Jones, J.-P.; Shevade, A. V.; Bugga, R. V. Transition metal phosphorous trisulfides as cathode materials in high temperature batteries. J. Electrochem. Soc. 2020, 167, 110512.
-
Wang, Y.; Zhou, Z.; Wen, T.; Zhou, Y.; Li, N.; Han, F.; Xiao, Y.; Chow, P.; Sun, J.; Pravica, M.; Cornelius, A. L.; Yang, W.; Zhao, Y. Pressure-driven cooperative spin-crossover, large-volume collapse, and semiconductor-to-metal transition in manganese(II) honeycomb gattices. J. Am. Chem. Soc. 2016, 138, 15751-15757.
-
Coradin, T.; Coupé, A.; Livage, J. Intercalation of biomolecules in the MnPS3 layered phase. J. Mater. Chem. 2003, 13, 705-707.
-
El-Meligi, A. A.; Al-Saie, A. M.; Al-Buflasa, H.; Bououdina, M. Formation of composite nanomaterial MnPS3 layered structure intercalated with pyridine. J. Alloys Compd. 2009, 488, 284-290.
-
Veerasubramani, G. K.; Park, M. S.; Choi, J. Y.; Lee, Y. S.; Kim, S. J.; Kim, D. W. Rational combination of an alabandite MnS laminated pyrrhotite Fe1-xS nanocomposite as a superior anode material for high performance sodium-ion battery. ACS Sustain. Chem. Eng. 2019, 7, 5921-5930.
-
Chen, X.; Li, W.; Xu, Y.; Zeng, Z.; Tian, H.; Velayutham, M.; Shi, W.; Li, W.; Wang, C.; Reed, D.; Khramtsov, V. V.; Li, X.; Liu, X. Charging activation and desulfurization of MnS unlock the active dites and electrochemical reactivity for Zn-ion batteries. Nano Energy 2020, 75, 104869.
-
Li, N.; Zhang, Y.; Cheng, R.; Wang, J.; Li, J.; Wang, Z.; Sendeku, M. G.; Huang, W.; Yao, Y.; Wen, Y.; He, J. Synthesis and optoelectronic applications of a stable p-type 2D material: α-MnS. ACS Nano 2019, 13, 12662-12670.
-
Köster, J.; Storm, A.; Ghorbani-Asl, M.; Kretschmer, S.; Gorelik, T. E.; Krasheninnikov, A. V.; Kaiser, U. Structural and chemical modifications of few-layer transition metal phosphorous trisulfides by electron irradiation. J. Phys. Chem. C 2022, 126, 15446-15455.
-
Komsa, H. P.; Kotakoski, J.; Kurasch, S.; Lehtinen, O.; Kaiser, U.; Krasheninnikov, A. V. Two-dimensional transition metal dichalcogenides under electron irradiation: defect production and doping. Phys. Rev. Lett. 2012, 109, no. 035503.
-
Gemmi, M.; Mugnaioli, E.; Gorelik, T. E.; Kolb, U.; Palatinus, L.; Boullay, P.; Hovmöller, S.; Abrahams, J. P. 3D electron diffraction: the nanocrystallography tevolution. ACS Cent. Sci. 2019, 5, 1315-1329.
-
Sombuthawee, C.; Bonsall, S. B.; Hummel, F. A. Phase equilibria in the systems ZnS-MnS, ZnS-CuInS2 , and MnS-CuInS2 . J. Solid State Chem. 1978, 25, 391-399.
-
Tan, H.; Verbeeck, J.; Abakumov, A.; Van Tendeloo, G. Oxidation state and chemical shift investigation in transition metal oxides by EELS. Ultramicroscopy 2012, 116, 24-33.
-
Yang, Z.; Peng, H.; Sun, J.; Perdew, J. P. More realistic band gaps from meta-generalized gradient approximations: only in a generalized Kohn-Sham scheme. Phys. Rev. B 2016, 93, 205205.