Real-time dynamics of Se atoms in CNTs observed by S/TEM
May 21, 2025 – A collaborative team from the University of Nottingham (UK), the SuperSTEM Laboratory in Daresbury (UK) and Ulm University (Germany) reports a controllable route to in‑situ nano‑engineering of elemental selenium. By confining Se inside boron nitride and carbon nanotubes and manipulating the filler with a low‑voltage electron beam, the scientists created one‑dimensional phases with optoelectronic properties that can be tuned on demand. This three‑in‑one use of the electron beam – as imaging probe, spectroscopic tool and nanoscale press – opens a pathway toward designer semiconductors for flexible nanoelectronics.
In a nutshell
Historically, selenium has played a pivotal role in solid-state physics. Experiments in the 1870s established photoconductivity1, revealed the photovoltaic effect in solids2, and led to the first solar cells3. Building on that legacy, researchers have spent decades tailoring Se allotropes because electronic and optical responses vary strongly with phase and dimensionality4,5.
The cylindrical cavities of carbon nanotubes (CNTs) and boron nitride nanotubes (BNNTs) act as Å-scale test tubes that template high-aspect-ratio nanomaterials while permitting atom-resolved transmission electron-microscopy (TEM) studies6,7. Previous work showed that a variety of semiconductors – SnSe8, CsPbBr39 and the elemental chalcogens S, Se and Te10–12 – adopt size-dependent structures when so confined. Now the researchers demonstrate how simply tuning the host diameter dictates the exact Se phase, from single linear chains to liquid-like assemblies.
Using aberration-corrected TEM (AC-TEM) and image simulations, the team mapped a one-dimensional phase diagram: a 0.73 nm CNT forces a single linear chain (l-Se); a slightly wider tube stabilises a trigonal helix (t-Se); still larger cavities host two parallel chains, and diameters >3 nm yield highly mobile, liquid-like Se. This systematic mapping had not been visualised directly before.
The scientists next exploited the electron beam itself as a nanoscale actuator. Under an 80 kV flux of 1.2 × 108 e− nm−2 s−1, Se-filled BNNTs developed side-wall vacancies that rearranged and contracted the tube diameter. As the “nano-vice” closed, encapsulated Se successively transformed from multi-helix to single-chain phases, live in the microscope. Vacancy propagation was accelerated by Se–B/N bonding, lowering the defect-formation barrier via the Bell–Evans–Polanyi principle29 – a behaviour not observed in CNT hosts, where Stone–Wales rearrangements dissipate strain30.
Band-gap mapping with ultra-low-loss monochromated STEM-EELS at 60 kV revealed a non-monotonic trend. Quantum confinement initially widens the gap, peaking at 2.45 eV for ~1.8 nm BNNTs hosting ≈20 helices of t-Se. Below that size, radial strain dominates, bending Se–Se bonds and narrowing the gap to 2.24 eV at 0.8 nm (four helices). This interplay explains the macroscopic orange colour of Se@BNNT powders.
Earlier device demonstrations showed that BNNT-confined semiconductors can function in nanoscale field-effect transistors and optoelectronic elements21,18. The present work adds the missing knob: a single instrument capable of imaging, spectroscopy and beam-driven fabrication, allowing dimensions, structure and bandgap to be tuned in real time. Such control is pivotal for future flexible photonics and quantum devices built one atom at a time.
Experiments performed on Se in the 1870s led to the discovery of photoconductivity1, the realization of the photovoltaic effect in solid semiconductors2, and its use as the photoactive component of the first solar cells3. Extensive research has since focused on producing and optimizing selenium-based semiconductors with defined allotropes, driven by the phase- and dimensionality-dependent nature of their electronic and optical properties4,5.
The cylindrical inner cavities of carbon nanotubes (CNTs) provide confined reaction environments for the templated growth of high aspect ratio nanomaterials and enable atomically resolved studies of such structures using transmission electron microscopy (TEM)6,7. This approach has been applied to diverse semiconducting materials including SnSe8 and CsPbBr39, as well as to the elemental chalcogens sulfur10, selenium11, and tellurium12.
Selenium was first encapsulated inside multi-walled carbon nanotubes (MWCNTs) in 199613, and has since been incorporated into CNTs with a wide range of diameters14. Fujimori et al. employed small-diameter double-walled CNTs (DWCNTs) to template single- and double-helix Se chains as narrow as 0.7 nm11. As the inner diameter of the host CNT increases, selenium adopts alternative conformations, including multiple trigonal Se (t-Se) chains15 or liquid-like t-Se phases exhibiting visible menisci14.
Recent studies have highlighted the advantages of boron nitride nanotubes (BNNTs) over CNTs as host materials. BNNTs offer improved capabilities for extending the fluorescence emission of encapsulated organic dyes16 and facilitate direct access to the electronic17 and optical18 properties of confined semiconductors.
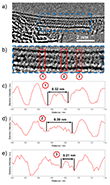
Aberration-corrected transmission electron microscopy (AC-TEM), supported by image simulations, was employed to investigate how the internal diameter of CNTs affects the structure and bonding of encapsulated selenium nanowires (Se NWs). The narrowest CNT studied (Fig. 1), with an inner diameter of 0.73 nm, hosts a linear atomic chain of selenium (l-Se), whose structure is shown in Fig. 1b. TEM simulations for l-Se within a CNT of matching diameter closely reproduce the experimental observations, revealing interatomic distances of 0.23 nm in the image and 0.24 nm in the model. The longest uninterrupted l-Se chain identified by AC-TEM spans at least 28 nm (Fig. 1a).
While previous Raman spectroscopic studies suggested the formation of l-Se chains within cancrinite nanochannels19, direct imaging evidence had been lacking. The structure observed here for l-Se under extreme confinement is consistent with findings on other chalcogens: both sulfur10 and tellurium12 have been shown to adopt linear chain configurations in narrow nanotube environments.
Additionally, the atomic spacing of selenium along the linear chains was measured as 0.23 nm in both experimental and simulated images (Fig. 2). As illustrated by the yellow square in Fig. 2c, two co-linear Se chains exhibit a staggered arrangement, likely resulting from the optimization of van der Waals interactions between adjacent atoms.
In some instances, the two co-linear l-Se chains were observed to twist along the axis of the CNT (Fig. 3), producing variations in their projected inter-chain distances. Similar co-linear atomic arrangements have previously been reported for tellurium encapsulated in 1.2 nm CNTs by Qin et al.21 The co-linear Se chains observed here may exhibit similar dynamic behavior to the double helices described by Fujimori et al.11, where rapid motion impeded atomic resolution imaging. In contrast, the present study focuses on stationary chain configurations, enabling precise determination of selenium atomic positions.
Due to van der Waals constraints, the maximum selenium-based structure that can be encapsulated in narrow CNTs consists of two t-Se chains. The internal diameter of the CNT governs the separation between these chains by mediating favorable interactions with the CNT walls22, resulting in increased inter-chain spacing as the CNT diameter increases. Larger diameters allow for the encapsulation of multiple t-Se chains (Fig. 4), as verified by matching simulated and experimental images.
Even in these wider CNTs, encapsulated Se NWs retain high translational and rotational mobility. Once the diameter is sufficient to host two l-Se or t-Se chains, the encapsulated selenium becomes less crystalline, resembling amorphous Se23. These mobile nanowires exhibit long-range disorder due to irregular twist axes (Fig. 3), while preserving short-range order, such as consistent interatomic spacing (Fig. 2) and defined inter-chain distances (Fig. 4c), which are in agreement with values for crystalline selenium.
This behavior parallels observations made by Laude and Fitton24, who characterized amorphous selenium not as a true amorphous material, but as a structurally disordered crystal. This interpretation is further supported by modeling studies that distinguish between static and dynamic disorder in similar systems25.
To further investigate the electron-beam-induced transformations, Se-filled BNNTs (Se@BNNT) were imaged under 80 kV AC-HRTEM at a controlled electron flux of 1.2 × 108 e−nm−2 s−1. During irradiation, single BNNTs showed the formation of sidewall defects (green arrow, Fig. 5a), which progressively expanded due to direct knock-on (DKO) damage. As previously demonstrated for empty BNNTs by Celik-Aktas et al.26 and Cheng et al.27, the removal of boron atoms can also destabilize neighboring nitrogen atoms, resulting in mass loss from the irradiated nanotube.
This phenomenon progresses faster in inner walls of multi-walled BNNTs due to their higher curvature and strain26,27. Prolonged imaging (400 s) revealed lines of contrast interpreted as chains of Se forming near BNNT sidewall defects (Fig. 5a). As this contrast proved reversible, it was attributed to a temporary suppression of translational and rotational motion of the encapsulated t-Se, rather than a permanent crystalline phase transformation.
Importantly, the authors observed that vacancy defects in BNNTs not only propagated but also restructured under continuous electron beam exposure, causing the BNNT diameter to shrink. This behavior mirrors the annealing and structural reorganization processes previously observed26,27. Here, the diameter contraction of BNNTs directly induces a phase transition from t-Se to l-Se in the encapsulated material.
A comparable beam-induced extrusion process has been reported for carbon nanotubes in Fe@MWCNT systems, although it required simultaneous heating to 600 °C28. As the BNNT diameter narrowed under irradiation, increasingly confined Se NWs formed, showing characteristic t-Se interchain distances consistent with those in narrow CNTs. Eventually, single linear Se chains (Fig. 5c,f) were observed in BNNTs with inner diameters as small as 0.8 nm, consistent with the 1D phase diagram derived from CNT-based systems.
The structural motifs—linear chains (Fig. 5c), seven t-Se chains (Fig. 5b), and smeared contrast in wide nanotubes (Fig. 5h)—mirror those found in CNTs. Although selenium interacts more strongly with BNNT walls22, these interactions are insufficient to prevent the mobility of the encapsulated chains. The authors expect the narrowed BNNTs formed during irradiation to be structurally stable26,27, suggesting that the Se nanowires templated under these dynamic conditions may resemble those observed in CNTs at ambient conditions.
The influence of Se on BNNT contraction under electron irradiation is likely linked to its effective bonding with boron or nitrogen dangling bonds at vacancy sites. This is supported by the emergence of well-defined t-Se chain contrast adjacent to newly formed defects (Fig. 5a). The formation of Se–B or Se–N bonds stabilizes these defects, thereby lowering the energy barrier for further defect formation in BNNTs according to the Bell–Evans–Polanyi principle29.
By contrast, selenium does not appear to promote defect formation in CNTs. This discrepancy may be attributed to the intrinsic ability of the carbon lattice to reorganize through the formation of five-membered rings and the Stone–Wales transformation, allowing strain redistribution and thus slowing diameter constriction under electron beam exposure30.
Ultra-low-loss electron energy-loss spectroscopy (EELS) enables the determination of optical bandgaps in nanoscale materials by probing their electronic excitations with high precision. When combined with aberration-corrected scanning transmission electron microscopy (AC-STEM) and highly monochromated beams, EELS achieves outstanding spatial and energy resolution, offering insight into local structure–property relationships31. This approach has been successfully employed in various systems, including empty carbon nanotubes (CNTs)32.
However, in CNTs, the presence of metallic or small-bandgap characteristics leads to overlapping van Hove singularities and π plasmon excitations, which obscure the electronic response of encapsulated materials32. In contrast, BNNTs offer an electronically transparent host matrix, enabling clearer spectral characterization.
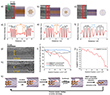
To prevent knock-on damage during data acquisition, high-angle annular dark-field (HAADF) AC-STEM imaging (Fig. 6a,c,e,g) and corresponding AC-STEM–EELS analysis (Fig. 6b,d,f,h) were conducted at an acceleration voltage of 60 kV. The internal diameters of the filled BNNTs studied exceeded 1.4 nm, a range which—according to the 1D Se phase diagram—favors the formation of nanowires (NWs) with similar crystalline disorder but differing in diameter.
EELS analysis revealed a maximum bandgap of 2.45 eV in a 1.8 nm diameter BNNT, where the encapsulated t-Se NW consists of approximately 20 helical chains (Fig. 6c,i, inset). This widening of the bandgap is attributed to quantum confinement effects33.
Interestingly, as the BNNT diameter was reduced from 1.8 nm to 0.8 nm, the observed bandgap narrowed to 2.24 eV, corresponding to a structure containing only four t-Se helices (Fig. 6g,i, inset). This trend highlights the complex interplay between structural dimensionality and optical properties in confined selenium phases.
In summary, recent studies by Qin et al.21 and Milligan et al.18 have demonstrated the successful integration of semiconductors encapsulated in boron nitride nanotubes (BNNTs) into field-effect transistors and optoelectronic nanodevices. These devices combine favorable form factors, chemical inertness, and high performance.
However, broader applicability hinges on the ability to understand and control the intrinsic properties of the confined semiconductors. The present study shows that the dimensions, atomic structures, and bandgaps of semiconducting nanowires within BNNTs can be modulated with precision using low-voltage aberration-corrected transmission electron microscopy (AC-TEM).
This control over key material characteristics paves the way for the targeted design and functionalization of future nanoscale electronic devices. The work also underscores the multifunctionality of the electron beam, which acts simultaneously as an imaging, spectroscopic, and nanofabrication tool—highlighting its central role in advanced nanomaterials research.
Resource:
Cull, W. J., Ramasse, Q. M., Biskupek, J., Rance, G. A., Cardillo-Zallo, I., Weare, B. L., Fay, M. W., Whitney, R. R., Scammell, L. R., Fernandes, J. A., Kaiser, U., Patanè, A., & Khlobystov, A. N. (2025).
Flexible Selenium Nanowires with Tuneable Electronic Bandgaps.
Advanced Materials, 2025, 2501821.
https://doi.org/10.1002/adma.202501821
-
W. Smith (1873). Selenium: its electrical qualities and the effect of light thereon. J. Soc. Telegraph Eng., 2, 31.
-
Adams, W. G., & Day, R. E. (1877). IX. The action of light on selenium. Phil. Trans. Roy. Soc. Lond., 167, 313–349.
-
Fritts, C. E. (1883). ART. LII. – On a new form of selenium cell, and some electrical discoveries made by its use. Am. J. Sci., 26(156), 465.
-
Bisht, N., Phalswal, P., & Khanna, P. K. (2022). Selenium nanoparticles: A review on synthesis and biomedical applications. Mat. Adv., 3(3), 1415–1431.
-
Al Jahdaly, B. A., Al-Radadi, N. S., Eldin, G. M., Almahri, A., Ahmed, M. K., Shoueir, K., & Janowska, I. (2021). Selenium nanoparticles synthesized using an eco-friendly method: dye decolorization from aqueous solutions, cell viability, antioxidant, and antibacterial effectiveness. J. Mater. Res. Technol., 11, 85–97.
-
Skowron, S. T., Chamberlain, T. W., Biskupek, J., Kaiser, U., Besley, E., & Khlobystov, A. N. (2017). Chemical reactions of molecules promoted and simultaneously imaged by the electron beam in transmission electron microscopy. Acc. Chem. Res., 50(8), 1797–1807.
-
Fung, K. L., Weare, B. L., Fay, M. W., Argent, S. P., & Khlobystov, A. N. (2023). Reactions of polyaromatic molecules in crystals under electron beam of the transmission electron microscope. Micron, 165, 103395.
-
Faulques, E., Kalashnyk, N., Slade, C. A., Sanchez, A. M., Sloan, J., & Ivanov, V. G. (2022). Vibrational and electronic structures of tin selenide nanowires confined inside carbon nanotubes. Synth. Met., 284, 116968.
-
Kashtiban, R. J., Patrick, C. E., Ramasse, Q., Walton, R. I., & Sloan, J. (2023). Picoperovskites: the smallest conceivable isolated halide perovskite structures formed within carbon nanotubes. Adv. Mat., 35(10), 2208575.
-
Fujimori, T., et al. (2013). Conducting linear chains of sulphur inside carbon nanotubes. Nat. Commun., 4(1), 2162.
-
Fujimori, T., dos Santos, R. B., Hayashi, T., Endo, M., Kaneko, K., & Tománek, D. (2013). Formation and properties of selenium double-helices inside double-wall carbon nanotubes: experiment and theory. ACS Nano, 7(6), 5607–5613.
-
Medeiros, P. V., et al. (2017). Single-atom scale structural selectivity in Te nanowires encapsulated inside ultranarrow, single-walled carbon nanotubes. ACS Nano, 11(6), 6178–6185.
-
Loiseau, A., & Pascard, H. (1996). Synthesis of long carbon nanotubes filled with Se, S, Sb and Ge by the arc method. Chem. Phys. Lett., 256(3), 246–252.
-
Dutta, D., Gope, S., Negi, D. S., Datta, R., Sood, A. K., & Bhattacharyya, A. J. (2016). Pressure-induced capillary encapsulation protocol for ultrahigh loading of sulfur and selenium inside carbon nanotubes: application as high performance cathode in Li–S/Se rechargeable batteries. J. Phys. Chem. C, 120(51), 29011–29022.
-
Kobayashi, K., & Yasuda, H. (2015). Structural transition of tellurium encapsulated in confined one-dimensional nanospaces depending on the diameter. Chem. Phys. Lett., 634, 60–65.
-
Allard, C., et al. (2020). Confinement of dyes inside boron nitride nanotubes: photostable and shifted fluorescence down to the near infrared. Adv. Mat., 32(29), 2001429.
-
van Bezouw, S., et al. (2018). Diameter-dependent optical absorption and excitation energy transfer from encapsulated dye molecules toward single-walled carbon nanotubes. ACS Nano, 12(7), 6881–6894.
-
Milligan, G. M., Cordova, D. L. M., Yao, Z. F., Zhi, B. Y., Scammell, L. R., Aoki, T., & Arguilla, M. (2024). Encapsulation of crystalline and amorphous Sb2S3 within carbon and boron nitride nanotubes. Chem. Sci., 15(27), 10464–10476.
-
Poborchii, V. V., Lindner, G. G., & Sato, M. (2002). Selenium dimers and linear chains in one-dimensional cancrinite nanochannels: Structure, dynamics, and optical properties. J. Chem. Phys., 116(6), 2609–2617.
-
Cherin, P., & Unger, P. (1967). The crystal structure of trigonal selenium. Inorg. Chem., 6(8), 1589–1591.
-
Qin, J. K., et al. (2020). Raman response and transport properties of tellurium atomic chains encapsulated in nanotubes. Nat. Electron., 3(3), 141–147.
-
Jishi, R. A., White, C. T., & Mintmire, J. W. (2000). Endohedral selenium chains in carbon, boron nitride, and BC2N nanotubes. J. Quantum Chem., 80(3), 480–485.
-
Chang, Y., Huang, L., Zhou, Y., Wang, J., & Zhai, W. (2022). Controlled localized phase transition of selenium for color-selective photodetectors. ACS Appl. Mater. Interfaces, 14(4), 5624–5633.
-
Laude, L. D., & Fitton, B. (1972). Effects of crystalline disorder on the electronic structure of selenium and tellurium. J. Non-Cryst. Sol., 8, 971–977.
-
Dittrich, B. (2021). On modelling disordered crystal structures through restraints from molecule-in-cluster computations, and distinguishing static and dynamic disorder. IUCrJ, 8(2), 305–318.
-
Celik-Aktas, A., Stubbins, J. F., & Zuo, J. M. (2007). Electron beam machining of nanometer-sized tips from multiwalled boron nitride nanotubes. J. Appl. Phys., 102(2), 024310.
-
Cheng, G., Yao, S., Sang, X., Hao, B., Zhang, D., Yap, Y. K., & Zhu, Y. (2016). Evolution of irradiation‐induced vacancy defects in boron nitride nanotubes. Small, 12(6), 818–824.
-
Sun, L., Banhart, F., Krasheninnikov, A. V., Rodriguez-Manzo, J. A., Terrones, M., & Ajayan, P. M. (2006). Carbon nanotubes as high-pressure cylinders and nanoextruders. Science, 312(5777), 1199–1202.
-
Cao, K., et al. (2018). Comparison of atomic scale dynamics for the middle and late transition metal nanocatalysts. Nat. Commun., 9(1), 3382.
-
Björkman, T., Gulans, A., Krasheninnikov, A. V., & Nieminen, R. M. (2012). Van der Waals bonding in layered compounds from advanced density-functional first-principles calculations. PRL, 108(23), 235502.
-
Rafferty, B., & Brown, L. M. (1998). Electronic structure: Wide-band, narrow-band, and strongly correlated systems-direct and indirect transitions in the region of the band gap using electron-energy-loss spectroscopy. Phys. Rev. B, 58(16), 10326–10337.
-
Hage, F. S., & Ramasse, Q. M. (2016). The low-loss spectrum of individual carbon nanotubes revisited at high energy resolution in real and momentum space. Microsc. & Microanal., 22(S3), 966–967.
-
Mohammad, N. S. (2014). Understanding quantum confinement in nanowires: basics, applications and possible laws. J. Phys. Condens. Matter, 26(42), 423202.