Atomic-Scale Transformation of Nickel Nanocrystals to Carbides Observed via TEM
May 30, 2025 - At high temperatures in carbon-rich environments, metallic catalysts like nickel can undergo carbonization – forming metal carbides – which leads to catalyst deactivation. Researchers from ShanghaiTech University in China, the University of Nottingham and the University of Leeds in the U.K., and Ulm University in Germany have now directly observed, in real time and at atomic resolution, how a nickel nanocrystal transforms into nickel carbide inside a carbon nanotube.
Nickel is widely used as a catalyst in various processes, for example in the dry reforming of methane or in the copolymerization of olefins [1, 2]. It can also serve as a low-cost alternative to palladium in cross-coupling reactions [3]. Moreover, nickel and other transition metals have long been used as catalysts for growing carbon nanotubes and graphene [4, 5, 6].
During catalytic operation at high temperatures, however, the strong bonding between carbon atoms and the metal surface often leads to the formation of metastable metal carbides, which can alter carbon nanostructure growth and ultimately deactivate the catalyst [7, 8]. Carbon can be chemically absorbed as strongly bound monolayers or accumulate as graphitic multilayers on the metal, blocking active sites and causing catalyst poisoning [7, 8]. The conversion of nickel into nickel carbide reduces the specific surface area, activity, and stability of the catalyst [9]. Minimizing such carbonization of nickel is important to maintain catalyst performance, but this requires a detailed understanding of the carbonization mechanism. Thus, real-time observation of the atomic-scale nickel carbonization process is crucial for developing the next generation of stable metal catalysts.
Previous studies have examined metal carbonization using in situ techniques like X-ray diffraction and spectroscopy [10] and through theoretical calculations [11]. However, the atomic-scale mechanism of metal carbonization has remained elusive [9]. In earlier works, the authors and colleagues demonstrated that metal nanoparticles can be confined inside carbon nanotubes, allowing their structural evolution and interactions with carbon to be directly imaged at the atomic level with low-voltage aberration-corrected TEM [12, 13, 14, 15, 16]. In the present study, this approach was applied to nickel nanocrystals to investigate their carbonization, see Figure 1 and Figure 2.
In the experiments, nickel nanocrystals of about 40 atoms were confined inside single-walled carbon nanotubes (~1.4 nm inner diameter). The 80 keV electron beam served both to image the sample and to stimulate carbonization by displacing carbon atoms from the nanotube lattice. These liberated carbon atoms were either ejected into the vacuum or captured by the nickel nanocrystal, which progressively became carburized. Using time-resolved HRTEM imaging, the researchers recorded the entire transformation of a Ni nanocrystal into Ni40C40 (nickel carbide) in real time.
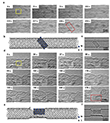
For example, Figure 3 shows a time series of TEM images in which a Ni nanocrystal (initially a ~4 nm fcc Ni40 cluster) gradually becomes carbonized and eventually cuts through the host nanotube over the course of 324 seconds. At the start (0 s), the Ni nanocrystal is pristine and separated from the tube wall by a ~0.3 nm van der Waals gap. By 82 s, carbon atoms have entered the Ni lattice (forming Ni40C20), and the particle bonds to the nanotube wall, creating defects in the carbon lattice. By 179 s, the nickel carbide has flattened into a quasi-two-dimensional form, and by 212 s significant damage is evident in the nanotube wall. Around 221 s, the NiC particle begins cutting into the upper wall of the SWNT, and by 277 s a large defect has formed where the nanotube is severed.
At the initial state (0 s), the Ni nanocrystal adopts a 4 × 5 × 4 face-centered cubic structure (40 Ni atoms) dictated by the nanotube’s ~1.5 nm inner diameter (Figure 3). There is no bonding between the metal and the carbon support initially, as a ~0.3 nm gap separates the Ni nanocrystal from the tube wall. Image simulations confirm that the nanocrystal is pure nickel metal at this stage.
As carbon begins to incorporate into the metal, the Ni lattice expands and its structure changes. By 82 s, the nanocrystal is partially carburized (approximately Ni40C20) while remaining crystalline (Figure 3). The experimental TEM image at 82 s was compared to simulations for various nickel carbide structures. The best match was obtained for NiC (nickel mono-carbide), indicating that carbon atoms occupy the octahedral interstices of the Ni lattice to form a NiC phase.
As the process continues, the NiC nanocrystal undergoes further transformation. By 125 s, it is strongly bonded to the nanotube, and increasing numbers of carbon atoms are extracted from the tube wall and incorporated into the NiC lattice. At 207 s, the nanocrystal is fully carbonized (approximate composition Ni40C40) and has thinned to only two atomic layers (Figure 4). TEM image analysis at 207 s, supported by simulations, shows an in-plane lattice expansion of about 12–15% relative to pure Ni, consistent with the formation of NiC. After the nanotube breaks, the tension on the NiC crystal is released: by 277 s, the particle reverts to a thicker (four-layer) structure (Figure 4e–h).
To test the reproducibility of the phenomenon, the team conducted multiple similar experiments. In all cases, Ni nanoparticles confined in nanotubes (with comparable initial sizes) gradually absorbed carbon from the host SWNT and were transformed into crystalline NiC, while the nanotubes became defective and were eventually cut by the NiC particles (Figure 5). Some Ni particles began as crystalline and others as amorphous clusters, but under 80 keV electron irradiation all of them underwent the carbonization process and induced the cutting of the nanotube. Notably, the force exerted by the breaking nanotube was sufficient to flatten the NiC cluster into an ultrathin 2D structure, suggesting a possible route to fabricate two-dimensional metal carbides. The real-time atomic-scale insights from this study shed light on the mechanism of nickel catalyst deactivation and could help in developing strategies to improve catalyst stability in carbon-rich environments.
Pu Yan, Dong Zhang, Wendi Zhang, Kaijun Sun, Meng Jin, Thomas W. Chamberlain, Andrei N. Khlobystov, Ute Kaiser, Yuan Hu, & Kecheng Cao (2025). Atomic-Scale Imaging of Transformation of Nickel Nanocrystals to Nickel Carbides in Real Time. ACS Nano. DOI: 10.1021/acsnano.5c06292
-
Huang, L., Li, D., Tian, D., Jiang, L., Li, Z., Wang, H., & Li, K. (2022). Optimization of Ni-based catalysts for dry reforming of methane via alloy design: A Review. Energy & Fuels, 36(10), 5102–5151.
-
Tan, C., & Chen, C. (2019). Emerging palladium and nickel catalysts for copolymerization of olefins with polar monomers. Angewandte Chemie, 131(22), 7268–7276.
-
Tasker, S. Z., Standley, E. A., & Jamison, T. F. (2014). Recent advances in homogeneous nickel catalysis. Nature, 509(7500), 299–309.
-
Rodríguez-Manzo, J. A., Terrones, M., Terrones, H., Kroto, H. W., Sun, L., & Banhart, F. (2007). In situ nucleation of carbon nanotubes by the injection of carbon atoms into metal particles. Nature Nanotechnology, 2(5), 307–311.
-
Jourdain, V., & Bichara, C. (2013).Current understanding of the growth of carbon nanotubes in catalytic chemical vapour deposition. Carbon, 58, 2–39. https://doi.org/10.1016/j.carbon.2013.02.046
-
Kim, K. S., Zhao, Y., Jang, H., Lee, S. Y., Kim, J. M., Kim, K. S., Ahn, J.-H., Kim, P., Choi, J.-Y., & Hong, B. H. (2009). Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature, 457(7230), 706–710. https://doi.org/10.1038/nature07719
-
Patera, L. L., Africh, C., Weatherup, R. S., Blume, R., Bhardwaj, S., Castellarin-Cudia, C., Knop-Gericke, A., Schloegl, R., Comelli, G., Hofmann, S., & Cepek, C. (2013). In situ observations of the atomistic mechanisms of Ni catalyzed low temperature graphene growth. ACS Nano, 7(9), 7901–7912.
-
Kimmel, Y. C., Xu, X., Yu, W., Yang, X., & Chen, J. G. (2014). Trends in electrochemical stability of transition metal carbides and their potential use as supports for low-cost electrocatalysts. ACS Catalysis, 4(5), 1558–1562.
-
Bayer, B. C., Bosworth, D. A., Michaelis, F. B., Blume, R., Habler, G., Abart, R., Weatherup, R. S., Kidambi, P. R., Baumberg, J. J., Knop-Gericke, A., et al. (2016). In situ observations of phase transitions in metastable nickel (carbide)/carbon nanocomposites. The Journal of Physical Chemistry C, 120(39), 22571–22584.
-
Sun, X., Yu, J., Cao, S., Zimina, A., Sarma, B. B., Grunwaldt, J.-D., Xu, H., Li, S., Liu, Y., & Sun, J. (2022). In situ investigations on structural evolutions during the facile synthesis of cubic α-MoC1–x catalysts. Journal of the American Chemical Society, 144(49), 22589–22598.
-
Magnin, Y., Zappelli, A., Amara, H., Ducastelle, F., & Bichara, C. (2015). Size dependent phase diagrams of nickel–carbon nanoparticles. Physical Review Letters, 115(20), 205502. https://doi.org/10.1103/PhysRevLett.115.205502
-
Cao, K., Biskupek, J., Stoppiello, C. T., McSweeney, R. L., Chamberlain, T. W., Liu, Z., Suenaga, K., Skowron, S. T., Besley, E., Khlobystov, A. N., & Kaiser, U. (2020). Atomic mechanism of metal crystal nucleus formation in a single-walled carbon nanotube. Nature Chemistry, 12(10), 921–928.
-
Cao, K., Chamberlain, T. W., Biskupek, J., Zoberbier, T., Kaiser, U., & Khlobystov, A. N. (2018). Direct correlation of carbon nanotube nucleation and growth with the atomic structure of rhenium nanocatalysts. Nano Letters, 18(10), 6334–6339.
-
Cao, K., Skowron, S. T., Stoppiello, C. T., Biskupek, J., Khlobystov, A. N., & Kaiser, U. (2020). Direct imaging of atomic permeation through a vacancy defect in the carbon lattice. Angewandte Chemie International Edition, 59(51), 22922–22927.
-
Cao, K., Zoberbier, T., Biskupek, J., Botos, A., McSweeney, R. L., Kurtoglu, A., Stoppiello, C. T., Markevich, A. V., Besley, E., Chamberlain, T. W., et al. (2018). Comparison of atomic scale dynamics for the middle and late transition metal nanocatalysts. Nature Communications, 9(1), 3382.
-
Zoberbier, T., Chamberlain, T. W., Biskupek, J., Suyetin, M., Majouga, A. G., Besley, E., Kaiser, U., & Khlobystov, A. N. (2016). Investigation of the interactions and bonding between carbon and group VIII metals at the atomic scale. Small, 12(12), 1649–1657.