Caught on camera: Chemical reactions 'filmed' at the single-molecule level.
March 28, 2017 - Chemists around the world look forward to seeing chemical transformations at the single-molecule level. Now an international team of scientists has published a paper about their observation of two chemical reactions with molecules of similar size and shape but with different chemical composition: octathi[8]circulene (OCT) and perchlorocoronene (PCC) which they caught on camera with atomic resolution using their new method named chemTEM.
Observation in a laboratory experiment by ensemble-averaging analytical techniques can only support a proposed mechanism, [1, 2] definitive information about reaction mechanisms however can only be provided by direct observation at the single-molecule level. The latest advances in aberration-corrected high-resolution transmission electron microscopy (AC-HRTEM) now provide such a way to study the mechanisms of chemical reactions. The combination of sub-angstrom spatial resolution with a larger overall field of view and high image capture rate has enabled imaging of chemical transformations at the molecular level. In the past, defect formation, metal atom entrapment in graphene, [3-5] restructuring in carbon nanotubes, [5-8] transformations of inorganic nanostructures, [9] as well as fascinating molecular motion [10-12] have already been demonstrated.
“TEM has the highest bandwidth for the temporal resolution of the available imaging techniques. In our chemTEM approach, we use the electron beam of the microscope both as a fast imaging tool and as a source of energy to stimulate chemical reactions,” says Prof. Andrei Khlobystov from the university of Nottingham, UK. “Such dual use of the e-beam allows us to trigger and record transformations of molecules as they occur without the need to introduce any additional energy source.”
Elastic interactions with the e-beam transfer the energy to the molecules. [13] This is an important advantage over methods such as non-contact AFM, which requires additional energy as heat, or so-called four-dimensional TEM (4D TEM), which requires energy from laser pulses. [14-23] 4D TEM is denoting a technique using correlated laser beam and e-beam pulses. Furthermore, the outstanding time resolution of the 4D TEM technique has been achieved at the expense of spatial resolution, as 4D TEM is able to resolve features on the scale of tens of nm at best, i.e., 100 times bigger than atomic dimensions, thus this method is precluded from imaging chemical reactions at the single-molecule level.
Observation of chemical reactions under the ChemTEM
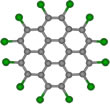
The circular shapes of perchlorocoronene (PCC, Fig. 1) and octathio[8]circulene (OTC, Fig. 2) are easily distinguishable in the TEM. Experimental images of PCC on graphene appear to be significantly blurred as compared to the simulated images of the stationary molecules (Fig. 1), indicating that the molecules remain mobile. However, the motion is not as fast as it could be expected for a medium-sized molecule on graphene, indicating that forces stronger than physisorption may exist between PCC and graphene under chemTEM conditions.
The molecule can exist in two different orientations with respect to the graphene surface: the face-on (appears as a circle) and the edge-on (appears as a line, Fig. 3a). The latter is stable only for a few seconds before the molecules returns back to the face-on orientation (Video 1). The abrupt transition of PCC from face-on to edge-on orientation is consistent with dechlorinated PCC covalently bonding to graphene (Fig. 3). Thus, the transformation is a chemical reaction rather than a physical effect of the e-beam. Further filming of PCC on graphene for extended periods of time indicates continuous fragmentation that does not lead to any stable products.
Video 1. ChemTEM video of reactions of PCC molecule on graphene under the 80 keV e-beam.
For further studies the scientists observed the same species inside a nanoscale reaction vessel - a SWNT - where the close-packed molecules are predisposed for intermolecular reactions. Entrapment in the nanotube can decelerate chemical transformations due to the extreme spatial confinement restricting the movement of molecules; therefore the SWNT provides ideal conditions for filming intermolecular reactions step-by-step, from the reactants all the way to the products.
As the electron dose increases, the adduct undergoes planarization followed by further increases in length transforming the original PCC into a ribbon-like product. The edges of the nano-ribbon are decorated with equidistantly positioned dark dots (Fig. 4), suggesting that atoms with a larger atomic number than carbon are terminating the edges of the structure.
“The transformation of discrete PCC molecules to the nano-ribbon over a series of metastable intermediates was recorded in a single experiment,” says Johannes Biskupek, who performed the TEM experiments. “We have continuously acquired frames of the reaction for the same molecules included in the nanotube, which means that the fate of each reacting molecule can be monitored precisely throughout the entire multi-step reaction process.”
In similar fashion to PCC on graphene, the kinetic energy of the e-beam transferred to an atom of PCC@SWNT drives bond dissociation in the molecule within the SWNT, but because the bond dissociation energy threshold depends on the orientation of the bond with respect to the direction of momentum transferred from the e-beam, [24] the energy thresholds for transformations of PCC inside nanotubes (in which the molecular plane is parallel to the e-beam) are expected to differ from those on graphene (molecular plane perpendicular to the e-beam).
The supporting DFT calculations revealed two major energy barriers (Fig. 5) for the chemical reaction. The rearrangement process is driven by the e-beam initiated formation of carbon radicals within the structure which releases four chlorine atoms, and the resultant species reorganizes to form the flat molecule C48Cl18, gaining full aromaticity (Fig. 5). This transformation proceeds via a series of metastable and hence transient intermediates which are too short-lived to be captured by AC-HRTEM. The AC-HRTEM imaging not only reveals the product of the multistep reaction, but also successfully captures an important intermediate, thus elucidating the two key steps of this complex chemical transformation.
OTC
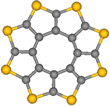
In order to demonstrate the generality of their approach, the scientists also investigated octathio[8]circulene (OTC, Fig. 2), a rare class of fully heterocyclic circulene molecule. Because sulfur atoms can bridge between two carbon atoms, OTC is expected to exhibit reaction pathways markedly different to PCC.
OTC (Fig. 2), the so-called sulflower molecule, [25] entrapped in nanotubes, forms stacks, OTC@SWNT (Fig. 6), that appear in AC-HRTEM images almost identical to PCC@SWNT. Time series imaging reveals that OTC molecules are more sensitive to the e-beam as the onset of their transformations takes place at a lower dose of the e-beam by a factor of 100 (Fig. 6). Neighboring PCC molecules require an interplanar angle of about 100° to undergo chemical reactions, which is not possible within the tight molecular stack in PCC@SWNT. Surprisingly OTC molecules are able to react with each other while still essentially constrained within the stack, forming a U-shaped adduct with an interplanar angle of only about 40° (Video 2, Fig. 6a).
Video 2. ChemTEM video of polycondensation of OTC inside carbon nanotube under the 80 keV e-beam.
Upon receiving further doses of electrons from the e-beam, the adduction of OTC undergoes planarization, thus gradually converting into a ribbon-like product inside the nanotube (Fig. 7a). The edge of the ribbon-like polymer has a higher contrast consistent with the presence of atoms with a higher atomic number than carbon along the edge (e.g., S atoms) and has a jagged appearance (Fig. 7a) which differs significantly from the atomically smooth edge of the nanoribbon formed from PCC under the same conditions.
Thanks to DFT calculations the exact shape of the C31S16 cycloadduct could be determined. The observed reaction pathways are consistent with the elimination of the S atom of the former thiophene ring followed by elimination of the under-coordinated sextet C atom (Fig. 7b, top) or these two processes in a reverse order (Fig. 7b, bottom), both leading to a planarized C30S15 product (Fig. 7b, Fig. 6a,b, last two frames). This process of radical addition, followed by C and S-atom elimination, and finally planarization can repeat again at either end of the C30S15 molecule and gradually converts the starting OTC compound to a ribbon-like polymeric product inside the nanotube (Fig. 7a).
AC-HRTEM images reveal that the polymer formed from OTC molecules has a complex undulating structure which cannot be described as either a solely zigzag or armchair edged nanoribbon because some sulfur atoms appear to have a role terminating the structure (in the thiophene ring) and while others act as bridging atoms (sulfide bridge) within the polymeric product (Fig. 8). This is in stark contrast to the perfect polyacene (zigzag) nanoribbon formed from PCC under the same conditions, which can be explained by an insufficient amount of carbon in this molecule.
“Up to now recording a ‘movie’ of intermolecular reactions, where chemical transformations of a single, uniquely identifiable molecule are continually followed from the starting reactant all the way to the final product, via a series of intermediates, is a formidable challenge,“ says Prof. Ute Kaiser, director of the SALVE project. “A series of breakthroughs in sample preparation, modelling and high resolution transmission electron microscopy (HRTEM) methods has now brought this dream to reality.”
Resource: Chamberlain, T. W., Biskupek, J., Skowron, S. T., Markevich, A. V., Kurasch, S., Reimer, O., Walker, K. E., Rance, G. A., Feng, X., Müllen, K., Turchanin, A., Lebedeva, M. A., Majouga, A. G., Nenajdenko, V. G., Kaiser, U. A., Besley, E., and Khlobystov, A. N. (2017). Stop-frame filming and discovery of reactions at the single-molecule level by transmission electron microscopy. ACS nano, 11: 2509., doi: 10.1021/acsnano.6b08228, [PDF], see also the supporting information.
Atkins, P., & Jones, L. (2002). Chemical principles: the quest for insight. 2nd ed.; W.H. Freeman & Co: New York, 2002.
Logan, S. R. (1996). Fundamentals of Chemical Kinetics. Longman Group Ltd.: London, 1996.
Kotakoski, J., Mangler, C., & Meyer, J. C. (2014). Imaging atomic-level random walk of a point defect in graphene. Nature Communications, 5: 3991.
Robertson, A. W., Lee, G. D., He, K., Yoon, E., Kirkland, A. I., & Warner, J. H. (2014). Stability and Dynamics of the Tetravacancy in Graphene. Nano Letters, 14: 1634-1642.
Rodríguez-Manzo, J. A., Cretu, O., & Banhart, F. (2010). Trapping of metal atoms in vacancies of carbon nanotubes and graphene. ACS Nano, 4: 3422-3428.
Rodríguez-Manzo, J. A., Terrones, M., Terrones, H., Kroto, H. W., Sun, L., & Banhart, F. (2007). In situ nucleation of carbon nanotubes by the injection of carbon atoms into metal particles. Nature Nanotechnology, 2: 307-311.
Sun, L., Banhart, F., Krasheninnikov, A. V., Rodriguez-Manzo, J. A., Terrones, M., & Ajayan, P. M. (2006). Carbon nanotubes as high-pressure cylinders and nanoextruders. Science, 312: 1199-1202.
Chamberlain, T. W., Meyer, J. C., Biskupek, J., Leschner, J., Santana, A., Besley, N. A., Bichoutskaia, E., Kaiser, U., & Khlobystov, A. N. (2011). Reactions of the inner surface of carbon nanotubes and nanoprotrusion processes imaged at the atomic scale. Nature chemistry, 3: 732-737.
Sloan, J., Matthewman, G., Dyer-Smith, C., Sung, A. Y., Liu, Z., Suenaga, K., Kirkland, A. I., & Flahaut, E. (2008). Direct imaging of the structure, relaxation, and sterically constrained motion of encapsulated tungsten polyoxometalate Lindqvist ions within carbon nanotubes. ACS Nano, 2: 966-976.
Koshino, M., Niimi, Y., Nakamura, E., Kataura, H., Okazaki, T., Suenaga, K., & Iijima, S. (2010). Analysis of the reactivity and selectivity of fullerene dimerization reactions at the atomi level. Nature chemistry, 2: 117-124.
Harano, K., Takenaga, S., Okada, S., Niimi, Y., Yoshikai, N., Isobe, H., Suenaga, K., Kataura, H., Koshino, M., & Nakamura, E. (2013). Conformational analysis of single perfluoroalkyl chains by single-molecule real-time transmission electron microscopic imaging. Journal of the American Chemical Society, 136: 466-473.
Khlobystov, A. N., Porfyrakis, K., Kanai, M., Britz, D. A., Ardavan, A., Shinohara, H., Dennis, T. J. S. & Briggs, G. A. D. (2004). Molecular Motion of Endohedral Fullerenes in Single‐Walled Carbon Nanotubes. Angewandte Chemie International Edition, 43: 1386-1389.
Turchanin, A., & Gölzhäuser, A. (2016). Carbon Nanomembranes. Advanced Materials, 28: 6075-6103.
de Oteyza, D. G., Gorman, P., Chen, Y. C., Wickenburg, S., Riss, A., Mowbray, D. J., Etkin, G., Pedramrazi, Z., Tsai, H.-Z., Rubio, A., Crommie, M. F., & Fischer, F. R. (2013). Direct imaging of covalent bond structure in single-molecule chemical reactions. Science, 340: 1434-1437.
Di Giovannantonio, M., El Garah, M., Lipton-Duffin, J., Meunier, V., Cardenas, L., Fagot Revurat, Y., Cossaro, A., Verdini, A., Perepichka, D., Rosei, F., & Contini, G. (2013). Insight into organometallic intermediate and its evolution to covalent bonding in surface-confined Ullmann polymerization. ACS Nano, 7: 8190-8198.
Hla, S. W., Bartels, L., Meyer, G., & Rieder, K. H. (2000). Inducing all steps of a chemical reaction with the scanning tunneling microscope tip: towards single molecule engineering. Physical Review Letters, 85: 2777.
Hulsken, B., Van Hameren, R., Gerritsen, J. W., Khoury, T., Thordarson, P., Crossley, M. J., Rowan, A. E., Nolte, R. J. M., Elemans, J. A. A. W., & Speller, S. (2007). Real-time single-molecule imaging of oxidation catalysis at a liquid–solid interface. Nature nanotechnology, 2: 285-289.
Zhou, H., Liu, J., Du, S., Zhang, L., Li, G., Zhang, Y., Li, G., Zhang, Y., Tang, B. Z., & Gao, H. J. (2014). Direct visualization of surface-assisted two-dimensional diyne polycyclotrimerization. Journal of the American Chemical Society, 136: 5567-5570.
Treier, M., Pignedoli, C. A., Laino, T., Rieger, R., Müllen, K., Passerone, D., & Fasel, R. (2011). Surface-assisted cyclodehydrogenation provides a synthetic route towards easily processable and chemically tailored nanographenes. Nature chemistry, 3: 61-67.
Riss, A., Paz, A. P., Wickenburg, S., Tsai, H. Z., De Oteyza, D. G., Bradley, A. J., Ugeda, M. M., Gorman, P., Jung, H. S., Crommie, M. F., Rubio, A. & Fischer, F. R. (2016). Imaging single-molecule reaction intermediates stabilized by surface dissipation and entropy. Nature Chemistry, 8: 678-683.
Lorenz, U. J., & Zewail, A. H. (2014). Observing liquid flow in nanotubes by 4D electron microscopy. Science, 344: 1496-1500.
Zewail, A. H. (2010). Four-dimensional electron microscopy. Science, 328: 187-193.
Van Der Veen, R. M., Kwon, O. H., Tissot, A., Hauser, A., & Zewail, A. H. (2013). Single-nanoparticle phase transitions visualized by four-dimensional electron microscopy. Nature chemistry, 5: 395-402.
Chamberlain, T. W., Biskupek, J., Skowron, S. T., Bayliss, P. A., Bichoutskaia, E., Kaiser, U., & Khlobystov, A. N. (2015). Isotope substitution extends the lifetime of organic molecules in transmission electron microscopy. Small, 11: 622-629.
Chernichenko, K. Y., Sumerin, V. V., Shpanchenko, R. V., Balenkova, E. S., & Nenajdenko, V. G. (2006). “Sulflower”: a new form of carbon sulfide. Angewandte Chemie International Edition, 45: 7367-7370.