Carbon nanotubes as electrically active nanoreactors for multi-step inorganic synthesis
June 03, 2016 - In a continuation of their studies of chemical reactions in CNT, scientists from UK, Germany and Japan, with participation of the SALVE project, were able to achieve a further breakthrough. In the journal JACS they report that they both have performed the first inorganic synthesis with well-defined clusters as reaction products as well as for the first time carried out a 2-step synthesis in a CNT. With a further improvement of the technology necessary to extract the reaction products from CNT, these methods can provide significant contributions to the synthesis of compounds for e. g. catalysis and electronic devices.
Single-walled carbon nanotubes (SWNT) are among the most effective and universal containers for molecules. They are used to produce unusual polymers [1], nanoribbons [2, 3], nanotubes [4, 5] or nanodiamonds [6]. The feed molecules are usually fullerenes [7] or organic molecules [4, 8, 9]. The structure of the macromolecular products is also determined by the confinement within the SWNT: the nanotubes function as nanoscale chemical reactors [10].
Considerable less research has been carried out into the chemical transformation of inorganic compounds in SWNT [11]. There are studies on the conversion of metal hexacarbonyl complexes M(CO)6 (where M is a transition metal) into metallic nanoclusters, however, as a result of the synthesis, the number of metal atoms per cluster varied in the range from 30 to 60 atoms. For these clusters, a variation of the atomic number also means a considerable variation in the properties [12]. As a result, the interaction of the cluster with the interior of the nanotubes is difficult to investigate [13] and the development of practical applications of metal nanoclusters in nanotubes is hardly possible [14]. All currently known inorganic reactions in SWNT produce either polymeric ionic crystals of [MxAy]n type, where M is a cation and A is an anion [15, 16], or discrete clusters with poorly defined size and shape, as in the case of the transition metals mentioned above.
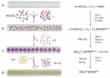
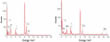
Now the research team from the Universities Nottingham, Ulm, Leeds and Warwick and the Institute of Advanced Industrial Science and Technology Japan has succeeded in specifically controlling also the reaction products of an inorganic synthesis by the inclusion in SWNT. In a chemical reaction they produced clusters with atomically precise stoichiometry. This was made possible by adding Iodine in addition to the transition metal carbonyl complexes (W(CO)6 or Mo(CO)6) as a source of the inorganic synthesis (Fig. 1). Since Iodine is significantly more directional than W or Mo, the clusters were more stable and in addition, the stronger bond between I2 and the CNT, wherein I2 attracts electron density from the host nanotube [17, 18], stabilized the clusters further. The withdrawal of electron density from the CNT's was inferred from Raman spectroscopy data (Fig. 2). The atomic structure of nanoclusters was imaged with temporally resolved Low-Voltage Aberration-Corrected Transmission Electron Microscopy (LV-AC-TEM) (Fig. 3) and the ratio of the elements was determined with energy dispersive X-ray (EDX) spectroscopy (Fig. 4).
For the study, scientists from the SALVE project at Ulm University assumed the task of imaging the starting material and the transformation steps with atomic resolution. The evaluation of the AC-HRTEM time series combined with structural modeling and TEM image simulation (Supporting information) resulted in a structure consisting of separate clusters similar to polyoxometalates [19] (Fig. 5) and an overall stoichiometry of M6I14, in good agreement with the result of the EDX analysis.
"In the HRTEM analysis, we found that the movements of the metal-iodine cluster were very fast, much faster than the movement of metallic clusters, which made the task much more complicated at first, especially in the case of Mo. For these particular structures, the Iodine atoms which are in contact with sp2 carbon atoms of nanotubes, did obviously not hamper the change of orientation," said SALVE Director Professor Ute Kaiser. "However, in the end, this circumstance was also beneficial, because due to the rotation of the clusters we could image all possible projections without tilting the sample."
Because of the very similar ionic diameter of W and Mo, n·[W6I14]2-@SWNT2n+ and n·[Mo6I14]2-@SWNT2n+ are hardly distinguishable in HRTEM images in the beginning. Over time, however, there are significant differences: [Mo6I14]2- is transformed into static polymerized structures (Fig. 6) and [W6I14]2- is in the state of discrete, highly mobile clusters (Video 1).
"This is another perfect example for the behavior of materials under the electron beam," said Biskupek, who performed the AC-HRTEM experiments. "The behavior can be explained by the different mass of the metal atoms. Since Mo is much lighter than W or I, it receives nearly twice as much energy by collisions with the electrons from the electron beam" (at 80 kV 1.97 eV for Mo, 1.03 eV for W, 1.00 eV for I).
The scientists analyzed in detail, how this affects the metal iodine nanoclusters (Fig. 7). An unsaturated atom is formed in the case of Mo, which achieves re-completion of his coordination environment by entering a connection with the neighboring clusters, which leads to polymerization of the structure. "Such a structure is totally unknown for Mo-I in the solid state," said Prof. Andrei Khlobystov, SALVE supporting member, who conducted the chemical analysis at the University of Nottingham. "The formation of [Mo6I12]n@SWNT in HRTEM is a combined result of the knock-on effect of the electron beam and the restriction effect of the nanotube, which allowed the development of this unusual structure."
The first multi-step reaction in a nanotube
The confinement inside the CNT has been used to specifically influence chemical reactions previously [1 - 6, 8, 9, 13, 20], but a 2- or multi-step synthesis was never carried out. It was also assumed that for this case, the metal-iodine cluster could potentially fill the entire cross section of the CNT (Fig. 3) and thus prevent any further chemical reaction. The nanotubes used, with a diameter of 1.4 nm, provide a tight container for the anionic [M6I14]2- cluster. By the addition of H2S gas, the scientists have now disproved this assumption (Figure 1c, d). They observed the subsequent conversion of the metal-iodine cluster in transition metal chalcogenide nanoribbons. The reaction proceeded efficiently and uniformly and without the loss of material from the SWNTs (Fig. 8, Video 2). As previously in a one-step synthesis in CNT [21], Molybdenium or Tungsten Sulfide nanoribbons could be synthesized within the CNT. Elemental analysis showed that iodine was almost completely removed and the metal:sulfur ratio was 1:2. The reaction with H2S gas begins at the end points of [M6I14]2- within the CNT and proceeds from both sides towards the interior of the nanotube. The width of the nanoribbons is strictly determined by the nanotube diameter and the sides have perfect Zigzag conformation in most cases (Fig. 8a). Occasionally some edge defects of the nanoribbons are found (Fig. 8b), which are to be expected at the points at which the nanoribbons growing from both sides meet. Due to the spatial restrictions, the defect cannot be compensated by rearrangement, but is stable over time.
Another important result of the study, which was found out by analysis of the Raman spectra, was that the positive charge of CNTs observed in the state of metal iodine nanoclusters decreased during the formation of the nanoribbons, in accordance with the fact that [MS2]n are electrically almost neutral (Fig. 1d). In the case of WS2 even a slight negative charge of the CNT was detected, which correlates with a slight difference of the valence band maxima of WS2 and MoS2 [22]. This demonstrated another feature of nanotubes: They not only limit the reaction in space but also electrons are made available when they are needed for a chemical reaction and are taken back from the reaction products when they are no longer required. So also unusual structures can be explained, as the observed separate metal-iodine compounds.
"With our comprehensive characterization and preparation opportunities we could again expand the range of synthesis in nanoscale containers. In the future, new electronically, optically and magnetically active inorganic nanostructures can possibly be produced in a similar manner," Kaiser said.
Resource: Botos, A., Biskupek, J., Chamberlain, T. W., Rance, G. A., Stoppiello, C., Sloan, J., Liu, Z., Suenaga, K., Kaiser, U. A., & Khlobystov, A. N. (2016). Carbon Nanotubes as Electrically Active Nanoreactors for Multi-Step Inorganic Synthesis: Sequential Transformations of Molecules to Nanoclusters, and Nanoclusters to Nanoribbons. Journal of the American Chemical Society, 138: 8175-8183, doi: 10.1021/jacs.6b03633, [PDF, Supporting information].
Pagona, G., Rotas, G., Khlobystov, A. N., Chamberlain, T. W., Porfyrakis, K., & Tagmatarchis, N. (2008). Azfullerenes encapsulated within single-walled carbon nanotubes. Journal of the American Chemical Society, 130: 6062-6063, doi: 10.1021/ja800760w
Talyzin, A. V., Anoshkin, I. V., Krasheninnikov, A. V., Nieminen, R. M., Nasibulin, A. G., Jiang, H., & Kauppinen, E. I. (2011). Synthesis of graphene nanoribbons encapsulated in single-walled carbon nanotubes. Nano letters 11: 4352-4356, doi: 10.1021/nl2024678
Chernov, A. I., Fedotov, P. V., Talyzin, A. V., Suarez Lopez, I., Anoshkin, I. V., Nasibulin, A. G., Kauppinen, E. I., & Obraztsova, E. D. (2013). Optical properties of graphene nanoribbons encapsulated in single-walled carbon nanotubes. ACS nano, 7: 6346-6353, doi: 10.1021/nn4024152
Lim, H. E., Miyata, Y., Kitaura, R., Nishimura, Y., Nishimoto, Y., Irle, S., Warner, J. H., Kataura, H., & Shinohara, H. (2013). Growth of carbon nanotubes via twisted graphene nanoribbons. Nature communications, 4: doi: 10.1038/ncomms3548
Shiozawa, H., Pichler, T., Grüneis, A., Pfeiffer, R., Kuzmany, H., Liu, Z., Suenaga, K., & Kataura, H. (2008). A catalytic reaction inside a single‐walled carbon nanotube. Advanced Materials, 20: 1443-1449, doi: 10.1002/adma.200701466
Nakanishi, Y., Omachi, H., Fokina, N. A., Schreiner, P. R., Kitaura, R., Dahl, J. E., Carlson, R. M. K., & Shinohara, H. (2015). Template synthesis of linear‐chain nanodiamonds inside carbon nanotubes from bridgehead‐halogenated diamantane precursors. Angew. Chem. Int. Ed., 54: 10802-10806, doi: 10.1002/anie.201504904
Chuvilin, A., Khlobystov, A. N., Obergfell, D., Haluska, M., Yang, S., Roth, S., & Kaiser, U. (2010). Observations of chemical reactions at the atomic scale: Dynamics of metal‐mediated fullerene coalescence and nanotube rupture. Angew. Chem. Int. Ed., 49: 193-196, doi: 10.1002/anie.200902243
Chamberlain, T. W., Biskupek, J., Rance, G. A., Chuvilin, A., Alexander, T. J., Bichoutskaia, E., Kaiser, U. A., & Khlobystov, A. N. (2012). Size, structure, and helical twist of graphene nanoribbons controlled by confinement in carbon nanotubes. ACS nano, 6: 3943-3953, doi: 10.1021/nn300137j
Chamberlain, T. W., Meyer, J. C., Biskupek, J., Leschner, J., Santana, A., Besley, N. A., Bichoutskaia, E., Kaiser, U. A., & Khlobystov, A. N. (2011). Reactions of the inner surface of carbon nanotubes and nanoprotrusion processes imaged at the atomic scale. Nature chemistry, 3: 732-737, doi: 10.1038/nchem.1115
Khlobystov, A. N. (2011). Carbon nanotubes: From nano test tube to nano-reactor. ACS nano, 5: 9306-9312, doi: 10.1021/nn204596p
Eliseev, A. A., Chernysheva, M. V., Verbitskii, N. I., Kiseleva, E. A., Lukashin, A. V., Tretyakov, Y. D., Kiselev, N. A., Zhigalina, O. M., Zakalyukin, R. M., Vasiliev, A. L., Krestinin. A. V., Hutchison, J. L., & Freitag, B. (2009). Chemical reactions within single-walled carbon nanotube channels. Chemistry of Materials, 21: 5001-5003, doi: 10.1021/cm803457f
Lu, Y., & Chen, W. (2012). Sub-nanometre sized metal clusters: from synthetic challenges to the unique property discoveries. Chemical Society Reviews, 41: 3594-3623, doi: 10.1039/C2CS15325D
Zoberbier, T., Chamberlain, T. W., Biskupek, J., Kuganathan, N., Eyhusen, S., Bichoutskaia, E., Kaiser, U. A., & Khlobystov, A. N. (2012). Interactions and reactions of transition metal clusters with the interior of single-walled carbon nanotubes imaged at the atomic scale. Journal of the American Chemical Society, 134: 3073-3079, doi: 10.1021/ja208746z
Chamberlain, T. W., Earley, J. H., Anderson, D. P., Khlobystov, A. N., & Bourne, R. A. (2014). Catalytic nanoreactors in continuous flow: hydrogenation inside single-walled carbon nanotubes using supercritical CO2. Chemical Communications, 50: 5200-5202, doi: 10.1039/C3CC49247H
Sloan, J., Kirkland, A. I., Hutchison, J. L., & Green, M. L. (2002). Structural characterization of atomically regulated nanocrystals formed within single-walled carbon nanotubes using electron microscopy. Accounts of Chemical Research, 35: 1054-1062, doi: 10.1021/ar010169x
Eliseev, A. A., Kharlamova, M. V., Chernysheva, M. V., Lukashin, A. V., Tretyakov, Y. D., Kumskov, A. S., & Kiselev, N. A. (2009). Preparation and properties of single-walled nanotubes filled with inorganic compounds. Russian Chemical Reviews, 78: 833, doi: 10.1070/RC2009v078n09ABEH004077
Song, H., Ishii, Y., Al-zubaidi, A., Sakai, T., & Kawasaki, S. (2013). Temperature-dependent water solubility of iodine-doped single-walled carbon nanotubes prepared using an electrochemical method. Physical Chemistry Chemical Physics, 15: 5767-5770, doi: 10.1039/C3CP50506E
Grigorian, L., Williams, K. A., Fang, S., Sumanasekera, G. U., Loper, A. L., Dickey, E. C., Pennycook, S. J., & Eklund, P. C. (1998). Reversible intercalation of charged iodine chains into carbon nanotube ropes. Physical Review Letters, 80: 5560, doi: 10.1103/PhysRevLett.80.5560
Sloan, J., Matthewman, G., Dyer-Smith, C., Sung, A. Y., Liu, Z., Suenaga, K., Kirkland, A. I., & Flahaut, E. (2008). Direct imaging of the structure, relaxation, and sterically constrained motion of encapsulated tungsten polyoxometalate Lindqvist ions within carbon nanotubes. ACS nano, 2: 966-976, doi: 10.1021/nn7002508
Chamberlain, T. W., Biskupek, J., Skowron, S. T., Bayliss, P. A., Bichoutskaia, E., Kaiser, U., & Khlobystov, A. N. (2015). Isotope substitution extends the lifetime of organic molecules in transmission electron microscopy. Small, 11: 622-629, doi: 10.1002/smll.201402081
Wang, Z., Li, H., Liu, Z., Shi, Z., Lu, J., Suenaga, K., Joung, S.-K., Okazaki, T., Gu, Z., Zhou, J., Gao, Z., Li, G., Sanvito, S., Wang, E., & Iijima, S. (2010). Mixed low-dimensional nanomaterial: 2D ultra-narrow MoS2 inorganic nanoribbons encapsulated in quasi-1D carbon nanotubes. Journal of the American Chemical Society, 132: 13840-13847, doi: 10.1021/ja1058026
Kang, J., Tongay, S., Zhou, J., Li, J., & Wu, J. (2013). Band offsets and heterostructures of two-dimensional semiconductors. Applied Physics Letters, 102: 012111, doi: 10.1063/1.4774090